Selenium levels in liver of Great White Egret (Ardea alba) from São Paulo metropolitan region, Brazil
Abstract
Selenium is an essential element for birds, although, when excessive it can become toxic and cause failure in their reproduction, mortality and deformities. The urban aquatic environment can be contaminated by Se originated from industrial processes, urban growth, agricultural practices, natural erosion and geochemical cycles. In this environment, Se can enter into the food chain, and the organisms of high trophic levels such as herons can accumulate over the time. In this study, selenium concentrations were determined in livers of Great White Egret (Ardea alba) from São Paulo Metropolitan Region (SPMR), the country largest urban center. The livers from the adult specimens surveyed were from specimens dead in the region during the period 2006-2009. These samples were ground, freeze-dried and homogenized for Se determinations by neutron activation analysis (NAA). Aliquots of each sample and synthetic Se standard were irradiated for 16 h under a thermal neutron flux of 2-4 x 1012 cm-2 s-1. After adequate decay time, the gamma ray measurements of samples and standards were carried out using an HPGe detector. In order to validate the analytical results of Se, NIST 1577b Bovine Liver certified reference material was analyzed and the results presented good precision and accuracy. Results of Se concentration in livers of Great White Egret (2.5-10.0 mg kg-1 dry weight) were compared with literature data. Comparisons made between the Se concentrations obtained for different genders of herons by applying nonparametric Mann-Whitney U test, at the significance level of 0.05, indicated that the females present lower concentrations. This low Se concentration found for females indicate the possibility of Se transference to the eggs, what can affect the reproductive process. These findings suggest the necessity of a systematic Se monitoring in habitats of Great White Egret.
Key words: Se concentration; toxic; genders; NAA.
Introduction
Selenium is an essential element for animals, although, in excess, it can cause clinical problems (Hoff et al. 1998, Franson 1999). According to De Luca-Abbott et al. (2001), selenium contamination is a serious problem for bird populations in various regions of the world due to its effect on the failure in reproduction, mortality and deformities in birds.
Selenium is present in some soils, but it can be a contaminant of urban aquatic environments when originated from industrial processes, urban growth, agricultural practices, natural erosion and geochemical cycles (Hoff et al. 1998, Franson 1999, Movalli 2000, Burger and Gochfeld 2001).
The diagnosis of Se poisoning is complicated due to its biological interaction with other elements, such as toxic metals Cd, Hg and Pb. Se interacts with these toxic metals promoting detoxification (Horai et al. 2007, Kim and Koo 2007). On the other hand, the absorption of high levels of Se makes it highly toxic for the development and reproduction of birds (Movalli 2000, Ohlendorf et al. 1990).
The vulnerability of birds to Se poisoning is primarily associated with their heavily contaminated habitats. Plants and invertebrates in contaminated aquatic system may accumulate Se in concentrations that are toxic to birds that eat them (Franson 1999). As the ardeids are at top of the aquatic food chain, through the contaminated food consumption, they are at the risk of Se bio-magnification (Franson 1999, De Luca-Abbott et al. 2001, Burger and Gochfeld 2001).
During the breeding season, the organisms of female birds can eliminate Se through the eggs and this high level of Se in the eggs can affect reproduction (Ohlendorf et al. 1990, Boncompagni et al. 2003). Consequently, it is of great concern to study Se concentrations among birds of different genders. Se concentrations in tissues of bird species are very scarce.
São Paulo Metropolitan Region (SPMR), the country largest urban center, presents increasing problems related to pollution, affecting the environment and the health of populations Fuga 2006). Another important risky factor is to find the occurrence of Great White Egret, generally, in areas considered polluted (Matarazzo-Neuberger 1994). This study was conducted to determine Se concentrations in livers of Great White Egret by neutron activation analysis (NAA). Differences in liver Se concentration between the genders of herons will be also evaluated.
Methods
Samples of Heron Livers and their Preparation for Analysis.
The number of Great White Egret (Ardea alba) liver specimens obtained for this study was limited to those found dead or injured in the SPMR and in the city of Sorocaba. Thirteen liver samples from egrets were supplied by the Technical Division of Veterinary Medicine and Wildlife Management (DEPAVE 3) /SVMA, São Paulo City Hall and two by the Municipal Zoo “Quinzinho de Barros”, Sorocaba. The causes of death for most birds were due to the disease caused by parasites or collisions with obstacles, such as trees, vehicles and others. The gender of birds was evaluated by gonadal examination (Gochfeld and Burger 1987). The collection of these specimens occurred in the period 2006-2009. Samples were collected under license from the Brazilian Institute of Environment and Natural Resources (IBAMA).
The livers of this species were stored at -20ºC after collection until their treatment for the analysis. Each liver sample was first cleaned by removing blood, then, cut in small pieces using a titanium knife. Next, it was freeze-dried and ground in an agate mortar to obtain a fine homogenous powder. A mean loss weight of about 73.3% was obtained in this drying process.
Procedure for Neutron Activation Analysis (NAA).
The synthetic standard of Se was prepared by pippeting 50 µl of the Se solutions onto a small sheet of Whatman No.40 filter paper, using an Eppendorf pipette The certified standard solution of Se provided by Spex Certiprep Chemical, USA, was used to prepare and dilute the Se solution. The filter sheets with the aliquots of standard Se solution were dried at room temperature inside a desiccator; then, they were placed into clean polyethylene envelopes. The mass of Se in the synthetic standard used in this study was 8.004 µg.
Aliquots of about 150-200 mg of each liver sample and reference material, after weighed, were placed in clean polyethylene envelopes and irradiated together with synthetic Se standard for 16 h, under a thermal neutron flux of about 2-4 x1012 n cm-2 s-1 of the IEA-R1 nuclear research reactor. Two series of measurements were carried out after 15 and 21 days of decay times, using an HPGe detector coupled to a gamma ray spectrometer. The gamma ray spectra were obtained using the MAESTRO software from EGG & Ortec and they were processed using the VISPECT2 computer program. The radioisotope measured was 75Se (264.66 keV, half life = 119.77 d) and the concentration was calculated by the comparative method.
The accuracy and precision of the Se results were verified by analyzing the certified reference material NIST 1577b Bovine Liver, provided by the National Institute of Standards & Technology, USA. Results obtained in these analyses showed a good precision, with relative standard deviation lower than 9.2% and good accuracy, with a relative error lower than 4.0%.
Results and Discussion
Concentrations of Se in the livers of Great White Egret obtained in this study are shown in Table 1.
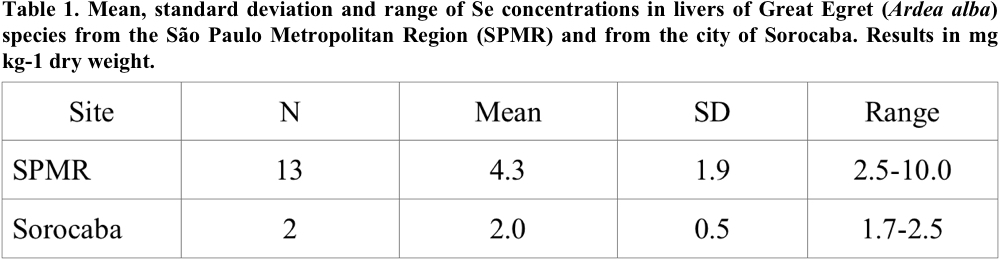
For most liver samples, Se concentrations were below 5.0 (mg kg-1 dry weight). Only one bird from SPMR presented concentration of 10.0 (mg kg-1 dry wt). Due to the small number of livers analyzed in the samples from the city of Sorocaba, comparison among the concentrations in birds from different habitats was not made.
There are few published data for Se in livers of ardeids. Most of the reported data are of feathers and eggs. Therefore, in this study, data from livers of other groups of birds were included for comparison with Se levels obtained in livers of Great White Egret in SPMR.
The Se concentration range (2.5-10.0 mg kg-1 dry wt) for Great White Egret livers from SPMR had the same order of magnitude (4.56-14.4 mg kg-1) of the data obtained from the Great White Egret livers in the industrial area of Haneda, considered polluted in Japan (Horai et al. 2007). Nevertheless, it is lower than the concentration range, (9.27-27.7 mg kg-1 dry wt), reported by Elliot et al. (1997), for seabirds in the Coast of Canada and for Ardea cinerea, from Japan (6.14-29.3 mg kg-1 dry wt) (Horai et al. 2007).
Se results obtained in this study (0.7 to 2.7 mg kg-1 wet weight) are very close to the values published for Ardea cinerea specimens, from Nottinghamshine North, UK where birds had deformities with multiple fractures of tarsus and tibia. Thompson et al. (2006) determined the levels of Se in specimens with these deformities and in normal individuals: they found no difference between the Se concentrations determined for these two groups. Therefore, these deformities were not associated with the toxicity of Se.
Ohlendorf et al. (1990) found Se in livers of the coots (2.3-9.8 mg kg-1 dry wt) and mallards (3.6-7.8 mg kg-1 dry wt) for birds from Volta, considered a control region. These Se values are of the same order of magnitude of those found for samples analyzed in this work. Therefore, we can consider that our results are within the range values reported as background in the literature.
There are few studies on the sensibility of ardeids to Se element. In the study of Smith et al. (1988) apud De Luca-Abbott et al. (2001), these authors examined the sensibility of black-crowned night herons to Se and concluded that herons were less sensitive to Se than mallards. However, the results from this study should be examined with caution since the number of samples was small and the hatching of control eggs was poor (De Luca-Abbott et al. 2001).
Selenium results obtained in livers of Great White Egret from SPMR are close to the values reported for other birds in which adverse effects of this element were not detected. However, as the sensibility among species and populations may differ (De Luca-Abbott et al. 2001), the Se effects can not be evaluated.
Due to the reduced data evaluated for Se and the contribution of few studies on the effects of Se contamination for the ardeids, in this preliminary study it was not possible to determine whether the levels of Se found in this study might be causing damage to the Great White Egret from SPMR.
In the Se determination in livers, the detection limit of 0.3 mg kg-1 was obtained according to Currie criterion (Currie 1968). The detection limit value is sufficiently far from the Se concentrations found in the liver of Great White Egret (Table 1), demonstrating that the NAA is a very appropriate technique for the Se determination in this type of tissue.
Comparative Study of the Levels of Se Found in the Livers from Specimens of Different Genders.
In this study, livers from 6 males and 6 females were analyzed and concentration means of 5.27 ± 2.35 mg kg-1 dry wt and 3.33 ± 0.7 mg kg-1 dry wt, respectively, were obtained. Fig. 1 shows the Se concentrations found in livers of male and female Great White Egret.
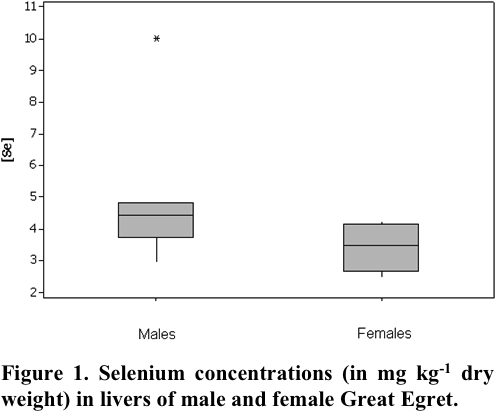
Since Se concentrations obtained for Great White Egret species did not show a normal distribution, a nonparametric statistical Mann-Whitney U test (Siegel 2006) was applied to a significance level lower than 0.05, in order to verify if there is a difference in Se concentration between genders. Statistical analysis of the data was conducted using SPSS software for Windows version 17.0 (Ferreira 2011), indicating significant differences for the Se concentration between males and females.
These findings may be related to physiological differences between the genders (Gochfeld and Burger 1987, Ayas 2007, Deng et al. 2007). There are several mechanisms for removing trace elements from the organisms of birds, such as direct elimination through feces or transference to the feathers. Besides, the female birds can transfer trace elements to the eggs, reducing the level of some elements in the tissues (Gochfeld and Burger 1987).
On the other hand, according to Ohlendorf et al. (1990), male and female birds, in general, have a similar rate of Se accumulation and loss in the liver, excepting during the breeding season when females eliminate Se through the eggs. However, these authors, in their preliminary studies found no differences between Se concentrations in livers of male and female birds from Kesterson Reservoir in California, where the Se contamination constituted a serious problem, causing mortality and anomalies in the population of birds. Movalli (2000), also found no significant differences in the Se concentrations between the genders in the analyses of feathers of falcons.
Literature data indicate that there is a controversy in relation to the differences of Se concentrations observed between the genders. These results demonstrate the need of this element determination in the Great White Egret species, focus of this work, to better elucidate this issue. Besides, periodic monitoring of Se in aquatic habitats can be an important strategy for future conservation of the wildlife.
Conclusions
The findings of this study showed that Se levels found in livers of the Great White Egret from SPMR are within the concentration range reported for birds from the control region.
Female Great White Egrets presented liver Se concentration lower than that found in the males, indicating possible transference of this element to the eggs in the breeding season. Therefore, liver analyses from females collected during the breeding season can clarify the transference of Se from tissues to the eggs.
Since Se can be toxic for birds, results obtained suggest the importance of a systematic monitoring of this element in their habitats.
Acknowledgements
The authors acknowledge the Technical Division of Veterinary Medicine and Wildlife Management (DEPAVE 3) /SVMA, Municipal Prefecture of São Paulo, and Municipal Zoo “Quinzinho de Barros”, Sorocaba for the liver samples provided for this study.
Literature Cited
Ayas, Z. 2007. Trace element residues in eggshells of grey heron (Ardea cinerea) and black-crowned night heron (Nycticorax nycticorax) from Nallihan Bird Paradise, Ankara-Turkey. Ecotoxicology 16: 347-352.
Boncompagni, E., A. Muhammad, R. Jabeen, E. Orvini, C. Gandini, C. Sanpera, C. Ruiz and M. Fasola. Egrets as Monitors of trace-metal contamination in Wetlands of Pakistan. Archives of Environmental Contamination and Toxicology 45: 399-406.
Burger, J. and M. Gochfeld. 2001. Metal levels in feathers of cormorants, flamingos and gulls from the Coast of Namibia in Southern Africa. Environmental Monitoring and Assessment. 69: 195-203.
Currie, L. A. 1968. Limits for qualitative detection and quantitative determination – application to radiochemistry. Analytical Chemistry 40: 586-593.
Deng, H., Z. Zhang, C. Chang and Y. Wang. 2007. Trace metal concentration in Great Tit (Parus major) and Greenfinch (Carduelis sinica) at the Western Mountains of Beijing, China. Environmental Pollution 148: 620-626.
Elliott, J. E. and A. M. Scheuhammer. 1997. Heavy metal and metallothionein concentrations in seabirds from the Pacific Coast of Canada. Marine Pollution Bulletin 34: 794-801.
Ferreira, A. R. 1999. SPSS - Manual de Utilização. Escola Superior Agrária de Castelo Branco. [online] Accessed 6 May 2011.
Franson, C. 1999. Selenium. Pp. 335-336, in Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Wild Birds. [online] Accessed 06 Jun 2001.
Fuga, A. 2006. Uso de liquens epifíticos no biomonitoramento da poluição atmosférica da Região Metropolitana de São Paulo. Dissertação (Mestrado), Instituto de Pesquisas Energéticas e Nucleares, São Paulo, Brazil.
Gochfeld, M. and J. Burger. 1987. Heavy metal concentrations in the liver of three duck species: Influence of species and sex. Environmental Pollution 45: 1-15.
Hoff, B., H. J. Boermans and J. D. Baird. 1998. Retrospective study of toxic metal analyses requested at a veterinary diagnostic toxicology laboratory in Ontario (1990-1995). The Canadian Veterinary Journal 39: 39-43.
Horai, S., I. Watanabe, H. Takada, Y. Iwamizu, T. Hayashi, S. Tanabe and K. Kuno. 2007. Trace element accumulations in 13 avian species collected from the Kanto area, Japan. The Science of the Total Environment. 373: 512-525.
Kim, J. and T. H. Koo. 2007. Heavy metal concentrations in diet and livers of Black-crowned Night Heron, Nycticorax nycticorax, and Grey Heron, Ardea cinerea, chicks from Pyeongtaek, Korea. Ecotoxicology 16: 411-416.
Luca-Abbott, S. B., B. F. Wong, D. B. Peakall, P. K. S. Lam, L. Young, M. H. W. Lam and B. J. Richardson. 2001. Review of effects of water pollution on the breeding success of waterbirds, with particular reference to ardeids in Hong Kong. Ecotoxicology 10: 327-349.
Matarazzo-Neuberger, W. M. 1994. Guildas, organização e estrutura da comunidade: análise da avifauna da represa Billings. Tese (Doutorado), Universidade de São Paulo, São Paulo, Brazil.
Metcheva, R., S. E. Teodorova, L. Yurukova and E. Nikolova. 2006. The penguin feathers as bioindicator of Antarctica environmental state. The Science of the Total Environment 362: 259-265.
Movalii, P. A. 2000. Heavy metal and other residues in feathers of Laggar Falcon, Falco biarmicus jugger, from six districts of Pakistan. Environmental Pollution 109: 267-275.
National Institute of Standards & Technology (NIST). 1991. Certificate of Analysis: Standard Reference Material 1577b Bovine Liver. National Institute of Standards & Technology, Gaithersburg, Maryland, USA.
Ohlendorf, H. M., R. L. Hothem, C. M. Bunck and K. C. Marois. 1990. Bioaccumulation of selenium in birds at Kesterson Reservoir, California. Archives of Environmental Contamination and Toxicology 19: 495-507.
Smith, G. J., G. H. Heinz, D. J. Hoffman, J. W. Spann and A. J. Krynitsky. 1988. Reproduction in black-crowned night-heron fed selenium. Lake and Reservoir Management 4: 175-180.
Siegel, S. 2006. Estatística não-paramétrica para ciências do comportamento. Bookman, São Paulo, Brazil.
Thompson, H. M., A. Fernandes, M. Rose, S. White and A. Blackburn. 2006. Possible chemical of skeletal deformities in grey heron nestling (Ardea cinerea) in North Nottinghamshine, UK. Chemosphere 65: 400-409.



