The Ardeid assemblages in the south coastal wetlands of Cuba #
Abstract
Cuba has 12 species of Ardeidae. Researchers have provided information about ardeids; however the spatial and temporal information is diverse and in some cases unpublished. This study evaluated the ardeid assemblage in 10 wetlands distributed along the southern coast of Cuba from May 2011 to March 2013. The data were grouped in three periods: spring migration, summer residence and fall migration. Specific richness ranged from 9-12 species per site. Ten species had an occurrence frequency above 80%. American Bittern (Botaurus lentiginosus) and Least Bittern (Ixobrychus exilis) were less frequent. The density ranged from 0.35-3.98 birds/ha. Tunas de Zaza and Jobabito wetland had the highest density (3.98 ± 0.39 birds/ha and 3.90 ± 0.54 birds/ha, respectively). Density values were significantly different among survey periods at some sites, e.g. Los Palacios, Las Salinas and La Zanja. Snowy Egret (Egretta thula) showed high values of proportional abundance (%) in most wetlands during the three survey periods. Cattle Egret (Bubulcus ibis), Reddish Egret (Egretta rufescens) and Great Egret (Ardea alba) had high contributions in some wetlands and this varied by period. Simultaneous assessment of the ardeid populations in the 10 wetlands increased our knowledge on their distribution patterns in Cuba, habitat preferences and importance of the migratory periods and habitat use.
Key words: ardeids; Cuba; range distribution.
# This paper was presented at the 1st Herons of the World Symposium at the 40th Anniversary Meeting of the Waterbird Society at New Bern, North Carolina, USA, 21-23 September 2016. Other papers from that Symposium will appear in future issues of the Journal of Heron Biology and Conservation, and Waterbirds.
Introduction
The Ardeidae family, with 17 genera and 62 species, is the largest within the Ciconiiformes (Kushlan and Hancock 2005). This group of birds has a world-wide distribution (del Hoyo et al. 1992) and is easy to recognize morphologically by its particular silhouette that includes bill, neck and long legs. At present, the image of an ardeid is increasingly used as a symbol of wetland conservation. Given their cosmopolitan distribution, the ardeids have a special potential to represent the value and role of wetlands and serve as sentinels of wetland health (e.g. bioindicators) (Mugica et al. 2006).
In Cuba, the ardeid family is represented by 12 species (Garrido and Kirkconnell 2010). With the exception of American Bittern (Botaurus lentiginosus), which is considered a migratory species, the rest of the family is classified as bimodal resident with resident and migrant populations (Llanes et al. 2002). The percentage of each species’ population in Cuba is unknown.
Several researches have provided information about ardeid ecology in both natural and anthropic Cuban ecosystems (Acosta et al. 1992, Sánchez and Rodríguez 2000, Mugica et al. 2001, Peña et al. 2012). However, the information is scattered, heterogeneous, unbalanced in time and space, and sometimes has not been published. Even though none of the species of ardeids present is threatened within Cuba, it is essential to have a complete assessment of the ardeid assemblages that use our wetlands and to maintain a monitoring program. As wetlands are one of the ecosystems vulnerable to climate change (Winter 2000), this information takes on greater value. The objective of this paper is to characterize the ardeid assemblages in natural wetlands in the southern coast of Cuba.
Methods
The research was carried out in eight natural wetlands distributed along the southern coast of Cuba. In the west was 1. National Park Guanahacabibes, 2. Faunal Refuge Punta Caribe and 3. Los Palacios; in the center was 4. National Park Las Salinas (Zapata Swamp), 5. Fauna Refuge Canales Hanábana (Zapata Swamp) and 6. Faunal Refuge Tunas de Zaza; in the east was 7 and 8. Fauna Refuge Monte Cabaniguán (two sites, see below) and 9 and 10. Fauna Refuge Delta del Cauto (two sites, see below) (Fig. 1). In the monitoring and data analysis, the eastern sites were divided in two. Faunal Refuge Monte Cabaniguán included 7. La Zanja and 8. Jobabito, while the Faunal Refuge Delta del Cauto was subdivided into 9. Mango and 10. Leonero. Thus, the results are given in terms of ten wetlands (Fig. 1).
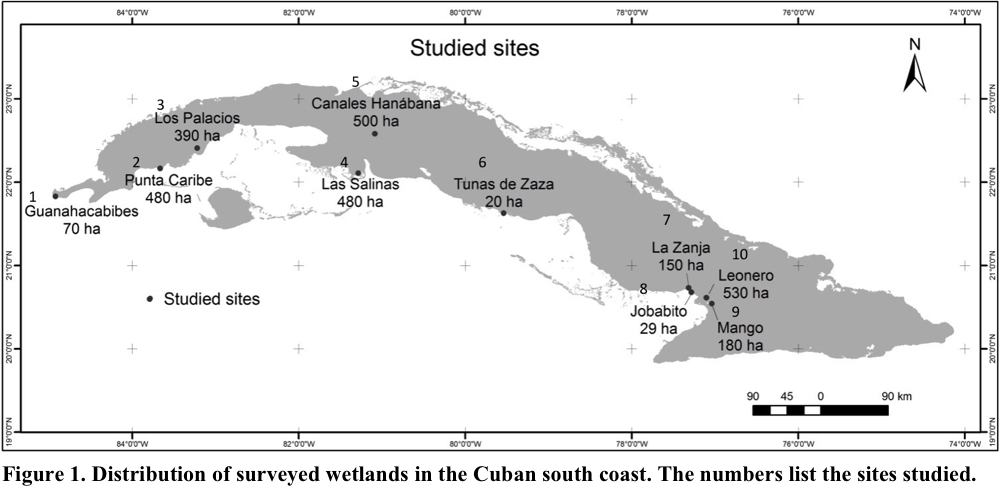
Sampling was done at the 10 sites during the period from May 2011 to November 2013; specifically, the counts were conducted in the months of February, March, May, June, October and November. Sampling days did not match among localities but the time was the same for all sites (0730-1130 h). In general, the sampling methods used were the point count and area search of Acosta et al. (2013). In both methods, all the birds were counted in a given area, the variation was that in the first method the counts were made from a fixed point and in the second were walking transects. In some locations, such as Punta Caribe and Las Salinas, there was a combination of both methods for different sectors within the area. Specifically, the search area was surveyed by walking or by boat. In the latter case, the sampled sites were Los Palacios, Canales Hanábana, Jobabito and Leonero. In all cases, a motor boat was used to reach the areas of the counts. Once at the sampling site, the boat was only rowed to minimize disturbance from the boat engine.
It is important to mention that the methods and sampling times used were not optimal for monitoring American Bittern, Least Bittern (Ixobrychus exilis) and Black-crowned Night-Heron (Nycticorax nycticorax), so these species are probably underestimated in our results.
The ardeid assemblage in each wetland was first characterized according to specific richness (species composition). This analysis was done for each wetland and each migratory/seasonal period. For that purpose, months were grouped in three periods: spring migration (February–March), summer residence/breeding season (May–June) and fall migration (October–November). The frequency of occurrence was calculated on the basis of presence or absence of the different species in the wetlands. Abundance (density) values were expressed in birds per hectare (birds/ha) and were calculated for each site and period. The data met the assumptions of normality and homogeneity of variance for parametric tests therefore; an Analysis of Variance (ANOVA) was used to compare the density of ardeids among periods in each wetland at a significance level of 0.05, with the program Statistica 8.0 (StatSoft, 2007). The proportional abundance (total number of individuals of a species among the total of individuals in the assemblage) was calculated for each species in the three survey periods to assess their representativeness at the site.
Results
Heron Distribution by Site
The specific richness recorded in the 10 wetlands varied between 9 and 12 species per wetland (Table 1). Canales Hanábana registered the largest number of ardeid species with 12; Punta Caribe, Leonero and Las Salinas had 10 species, Guanahacabibes, La Zanja and Mango had 9 species and the rest of the sampled sites 11 species.
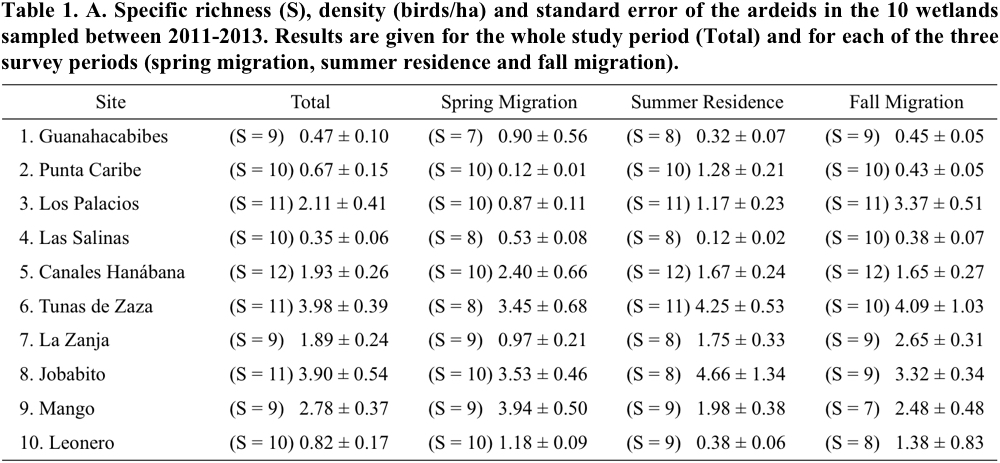
Of all species, ten showed a very high frequency of occurrence. The species with 100% frequency were Great Egret (Ardea alba), Great Blue Heron (Ardea herodias), Snowy Egret (Egretta thula), Little Blue Heron (Egretta caerulea), Tricolored Heron (Egretta tricolor) and Green Heron (Butorides virescens). Other species with high frequency values (90%) were Reddish Egret (Egretta rufescens), Cattle Egret (Bubulcus ibis), Black-crowned Night-Heron and Yellow-crowned Night-Heron (Nyctanassa violacea). The species with the lowest values of frequency were Least Bittern and American Bittern (50% and 10%, respectively).
In general, the total density of birds varied between 0.35 and 3.98 birds/ha (Table 1). The wetlands that had the highest densities of birds were Tunas de Zaza (site 6) and Jobabito (8). They were followed by Mango (9), Los Palacios (3), Canales Hanábana (5) and La Zanja (7). The rest of the wetlands had total densities of 0.38 birds/ha or lower.
Heron Distribution by Season
The species richness showed slight variations by season (spring migration, summer residence and fall migration) (Table 1). At 40% of sites, species richness was slightly lower during spring than fall migration and summer residence migration owing to the absence of one or two species. The greatest variations among periods were detected in the density of birds. Statistical comparisons revealed significant differences among periods in: Punta Caribe (F = 17.75, p ˂ 0.001, df = 2); Los Palacios (F = 10.47, p = 0.004, df = 2); Las Salinas (F = 11.75, p ˂ 0.001, df = 2); La Zanja (F = 6.87, p = 0.009, df = 2); Mango (F = 5.49, p = 0.032, df = 2) and Leonero (F = 6.57, p = 0.017, df = 2). In general, there were differences in the periods with highest density values. For example, the most important period in Las Salinas and Mango was spring migration; in Punta Caribe it was the summer residence, and in Los Palacios and La Zanja it was the fall migration (Table 1).
The proportional abundance of the ardeid species in the three periods is shown in Table 2. During the spring migration, Snowy Egret was among the most numerous species (high proportional abundance values) at all wetlands. Particularly in Guanahacabibes (Site 1), Los Palacios (3), Jobabito (8) and Mango (9), Snowy Egret was the species with the highest values. In the other localities, it was the second most numerous species. In Canales Hanábana (Site 5), Tunas de Zaza (6) and Leonero (10), Cattle Egret showed the highest proportional abundance values.
In summer residence, some species showed patterns of abundance similar to those observed in spring migration (Table 2). For example, Snowy Egret was among the most numerous in all localities being the most representative in Punta Caribe (Site 2), Los Palacios (3), La Zanja (7), Jobabito (8) and Mango (9). The Cattle Egret maintained a high proportional abundance values in Canales Hanábana (5) and Tunas de Zaza (6) (Table 2). It is interesting to mention in this period the higher proportional abundance of the Reddish Egret in Las Salinas (4); and the values of Green Heron in Leonero (10), Guanahacabibes (1) and Canales Hanábana (5).
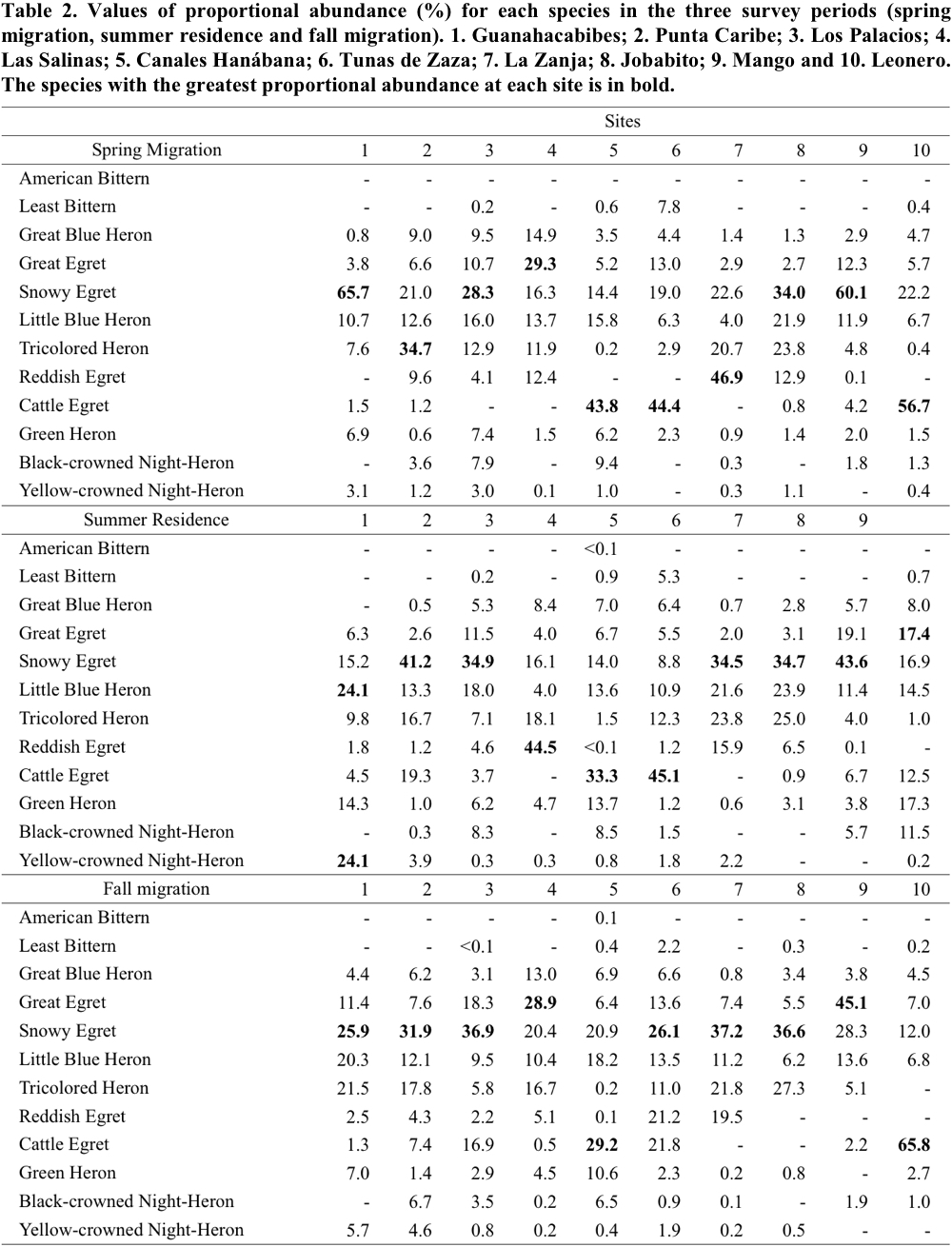
Finally, in the fall migration, proportional abundance values were similar to those recorded in previous periods; Snowy Egret was found again among the most numerous species. With the exception of Las Salinas (Site 4), Canales Hanábana (5), Mango (9) and Leonero (10), it was the species of greatest contribution in the remaining wetlands (Table 2). Similarly, Cattle Egret remained as the species most representative in Canales Hanábana (5) and Leonero (10). In this period, Great Egret was the species of greatest abundance in the localities of Las Salinas (4) and Mango (9).
Discussion
In general, the results provide important information confirming the wide distribution and abundance of ardeids in southern Cuba. The study sites, regardless of their size and particular characteristics, displayed high species richness. Canales Hanábana and Leonero were the sites where there was a predominance of freshwater habitats. However, given the general character (both in habitat and diet) of most of the ardeids, they showed a high occurrence frequency. In the same way, based on the total density values, no pattern was found regarding habitat use. Among the sites with the highest density were: small sites (Tunas de Zaza and Jobabito); large sites (Los Palacios y Canales Hanábana); sites with predominance of freshwater habitats (Canales Hanábana) and sites with predominance of saline and hypersaline habitats (Tunas de Zaza, Mango, Los Palacios).
The two bitterns were the less frequent and abundant species. They restricted their typical habitat to grasslands, rice fields and swamp grasslands (Denis et al. 2002). In addition, American Bittern was a very rare winter resident (Garrido and Kirkconnell 2010). Although the sampling time was not optimal for the monitoring of Black-crowned Night-Heron, due to their nocturnal habits (Kushlan and Hancock 2005), the species showed a high frequency of occurrence. It is likely that these observations were made in the early hours of the day when the individuals were returning to their roosting sites.
Given the bimodal residency category of all ardeids (except the American Bittern) in Cuba, species density was expected to vary; but not the species richness. Within the sites, the variation in species richness among periods was only two species. Meanwhile, the differences in density indicated that there was a seasonal variation in the use of the wetlands, likely due to resources available for feeding and breeding, i.e. some sites can be used for both feeding and reproduction, and others only for feeding. Punta Caribe, Canales Hanábana and Jobabito are sites where breeding colonies were registered within the sampled area (S. Aguilar unpublished data). Some species, like Green Heron in Canales Hanábana, increase their proportional abundance during the summer residence which corresponds to the breeding period of the ardeids. In sites used only for feeding like Las Salinas and Guanahacabibes, the density of birds may decrease during the reproductive season, when waterbirds should be feeding at sites closest to their breeding colony. But, in turn, populations at these feeding sites may increase in number during autumnal migration when birds return from the breeding sites in others parts of Cuba and the North American mainland.
It is also well known that water depth is one of the most important factors that affect habitat use in ardeids (Strong et al. 1997, Bancroft et al. 2002, Raposa et al. 2009, Lantz et al. 2011). Specifically in this group, the access to habitat for foraging is limited by leg length. In general, hydrological conditions within the natural wetlands are influenced by tidal cycle, rainfall, and/or wind action. It is likely that at certain times of the year, some of the wetland sites did not have adequate conditions for ardeids, either because of elevated water depth or because they were dry. Although water depth has been described by several researchers as a determinant in the use of habitat by waterbirds, little is known in Cuba about the magnitude of its influence.
Snowy Egret was one of the species that showed higher contributions in most locations and periods of study. In Cuba, Snowy Egret is widely distributed and is known to consume a wide variety of prey (Denis et al. 2002, Denis and Jiménez 2009). It is also considered the most social, commonly found in flocks (Caldwell 1981, Master 1992, Kushlan and Hancock 2005). All these elements could explain why this species showed a high contribution in almost all the 10 wetlands.
Other species with interesting results were Cattle Egret, Reddish Egret and Great Egret. Within the ardeids, Cattle Egret is among the most abundant species in North America with a widespread distribution (Kushlan and Hancock 2005). The species was first recorded in Cuba in the 1950’s, and since then, it has gradually become an abundant in natural and anthropic wetlands mainly agroecosystems (Denis et al. 2002). Its high values of proportional abundance in Canales Hanábana, Tunas de Zaza and Leonero are related to the proximity of the study area of each wetland to agricultural areas.
Reddish Egret is a coastal marine species that rarely uses interior areas (Kushlan and Hancock 2005). Despite it’s relatively high frequency of occurrence, only it showed high proportional abundance values in Las Salinas and La Zanja. Both of these wetlands include extensive areas of coastal lagoons that are used by Reddish Egrets to forage; they are also sites where breeding colonies have been identified in nearby areas (González et al. 2016).
The high values of Great Egret during fall migration may be due to the incorporation of migratory populations, or by populations returning from their breeding sites and their recruitment. So far it is unknown what percentage of the population of each heron species is in one or another category (migratory population or resident population). Further studies are needed in this regard through the monitoring of individually banded birds.
Acknowledgements
This paper benefited from research projects funded by GEF-PNUD, Whitley Fund for Nature and Caribbean Waterbirds Census, BirdsCaribbean. In addition, comments and suggestions from Chip Weseloh and Clay Green have greatly improved the paper.
Literature Cited
Acosta, M., J. Morales, M. González and L. Mugica. 1992. Dinámica de la comunidad de aves de la playa La Tinaja, Ciego de Ávila, Cuba. Ciencias Biológicas 24: 44-56.
Acosta, M., L. Mugica and S. Aguilar. 2013. Protocolo para el monitoreo de aves acuáticas y marinas. National Center of Protected Area, Havana, Cuba.
Bancroft, G. T., D. E. Gawlik and K. Rutchey. 2002. Distribution of wading birds relative to vegetation and water depths in the northern Everglades of Florida, USA. Waterbirds 25: 265-277.
Caldwell, G. S. 1981. Attraction to tropical mixed-species heron flocks: proximate mechanism and consequences. Behavioral Ecology and Sociobiology 8: 99-103.
del Hoyo, J., A. Elliott and J. Sagatal (eds.). 1992. Handbook of the birds of the world. vol. 1. Lynx Edicions, Barcelona, Spain.
Denis, D. and A. Jiménez. 2009. Nestling diet in five species of herons and egrets in Birama Swamp, Cuba. Journal of Caribbean Ornithology 22: 26-31.
Denis, D., M. Acosta, A. Jiménez, O. Torre and A. Rodríguez. 2002. Las zancudas. Pp. 128-136 in Aves de Cuba (H. González, ed.). UPC Print, Vaasa, Finland.
Garrido, O. and A. Kirkconnell. 2010. Aves de Cuba. Cornell University Press, Ithaca, New York, U.S.A.
González, A., A. Jiménez, L. Mugica, M. Acosta, I. García-Lau, R. Castro, M. López, J. M. de la Cruz, A. Pérez, Z. Hernández and S. Aguilar. 2016. Current status of Reddish Egret (Egretta rufescens) in Cuba. Waterbirds 39: 1-12.
Kushlan, J. A. and J. A. Hancock. 2005. The herons. Oxford University Press, New York, New York, U.S.A.
Lantz, S., D. Gawlik and M. Cook. 2011. The effects of water depth and emergent vegetation on foraging success and habitat selection of wading birds in the Everglades. Waterbirds 34: 439-447.
Llanes, A., H. González, B. Sánchez and E. Pérez. 2002. Lista de las aves registradas para Cuba. Pp. 147-155 in Aves de Cuba (H. González, ed.). UPC Print, Vaasa, Finland.
Master, T. L. 1992. Composition, structure, and dynamics of mixed-species wader aggregations in a southern New Jersey saltmarsh. Colonial Waterbirds 15: 149-157.
Mugica, L., M. Acosta and D. Denis. 2001. Dinámica temporal de la Comunidad de aves asociada a la arrocera Sur del Jíbaro. Biología 15: 86-96.
Mugica, L., M. Acosta and D. Denis. 2006. Conservando las aves acuáticas. Capítulo VII. Pp. 136-159 in Aves Acuáticas en los Humedales de Cuba (Mugica et al., eds.). Ciéntífico-Técnica, La Habana, Cuba.
Peña, C., J. W. Wiley, F. Ocaña, A. Vega, N. Navarro, S. Sigarreta and P. A. González. 2012. The avifaunal composition in the Río Mayarí Delta, northeastern Cuba. Journal of Caribbean Ornithology 25: 7-14.
Raposa, K. B. R., A. McKinney and A. Beaudette. 2009. Effects of tide stage on the use of salt marshes by wading birds in Rhode Island. Northeastern Naturalist 16: 209-224.
Sánchez, B. and D. Rodríguez. 2000. Avifauna associated with the aquatic and coastal ecosystems of Cayo Coco, Cuba. El Pitirre 13: 68-75.
StatSoft, Inc. 2007. STATISTICA version 8.0. [online].
Strong, A. M., G. T. Bancroft and S. D. Jewell. 1997. Hydrological constraints on Tricolored Heron and Snowy Egret resource use. Condor 99: 894-905.
Winter, T. C. 2000. The vulnerability of wetlands to climate change: a hydrologic landscape perspective. Journal of the American Water Resources Association 36: 1-7.



