Great Blue Heron
Ardea herodias (Linnaeus)
Ardea herodias Linnaeus, 1758. Syst. Nat. ed. 10(1), p. 143: America = Hudson’s Bay.
Subspecies: Ardea herodias occidentalis Audubon, 1853: Keys near Key West, Florida; Ardea herodias wardi Ridgway, 1882: Oyster = Estero Bay, Florida; Ardea herodias fannini Chapman, 1901: Skidegate, Graham Island, in Queen Charlotte Islands; Ardea herodias cognata Bangs, 1903: Indefatigable Island I. (Santa Cruz), Galapagos.
Other names: Blue Crane, Blue Heron, Great Blue, Great White Heron (white birds), Würdemann’s Heron (dark-white intermediate), Blue Gaulin, Arsnicker in English; Garza Azulada, Garza Azul Mayor, Garzón, Garzón Azul, Garzón Cenizo, Garzón Azulado, Garcilote, Garcilote Ceniciento, Garzón Blanco (white birds) in Spanish; Grand héron, Krabye nwa, lajirond, Crabier bleu, Crabier radar, Crabier noir in French, Haiti; Amerikaanse blauwe reiger in Dutch; Kanadareiher in German.
Description
The Great Blue Heron is a large, dimorphic heron. Dark birds are dark grey heron with chestnut thighs and a white cap over a black eye stripe. Light birds are all white.
Adult: The dark form of the Great Blue Heron is basically a grey blue bird. It has a white crown, cheeks and throat and a black stripe running from the eye, along side of the head merging into long black plumes. The plumes and white crown together form an erectile crest. The irises are yellow, and in nonbreeding the lores are a dark dull green, often surrounding a small yellow spot or line near the base of the bill. The long, deep bill is yellow, most often with a dusky top to the upper bill. The neck is grey, often with a chestnut to vinous tinge on the back and sides. The throat is vertically striped grey and white. The back is blue grey. At rest, a patch at the apparent “shoulder” (the epaulette) is black with chestnut toward the back. The undersides are light grey with black stripes. Thigh feathers are a distinctive chestnut. In flight, it has two-tone upper wings, darker behind, lighter in front, with patches of pale chestnut feathers at the bend in the wing (as field characters these are often called “headlights”). The lower legs are green brown, the upper legs often shading to pink. The white morph is all white. The legs are pale cream yellow and lores very pale blue. The bill is yellow, often with a dusky top.
During breeding the plumes achieve full development. The courtship crest incorporates the several long black head plumes. When raised the crest is black with a white center. The elongated throat and neck plumes are light grey with vinous wash. The similarly elongated back plumes also are light grey, contrasting with the darker back. The bill takes on an orange color near the base and more yellow to the tip, turning darker after courtship. The legs also take on an orange red color. These color changes may vary geographically as they are not reported in fannini (Butler 1992). The lores become blue green to blue tending to yellow at the base of the bill. In the white form, the plumes are all white, and the bill and legs turn very pink. The lores turn blue.
Variation: The sexes have similar plumage but females are about 10% smaller. Some individuals, especially in southern Florida, appear to be intermediate between the dark and white morphs, having pale body plumage and a white head and light neck. These birds (the Würdemann’s Heron) have been subject of discussion for over a century (Cory 1886, 1887). They likely are the result of parental or ancestral pairings of white and dark birds.
Subspecies of Great Blue Heron differ primarily in plumage shade and in size. The western race fannini has substantially darker dorsal coloration, longer wings, and a shorter bill than herodias. The southern race wardi is markedly larger and paler than herodias. The tropical races, Galapagos cognata and the dark form of the Caribbean occidentalis, are paler. Occidentalis has a high proportion of white morph birds, which are largest of the Great Blue Heron populations, being heavier by up to 10% and with distinctly heavier bills
Juvenile: Juveniles are darker than the adults and have a variably dark crown, lacking the white center and plumes of the adult. Soft parts are duller; the upper bill is slaty and lower bill yellow. The irises are yellow and the lores are dark with yellow interior. Juveniles have much more ventral striping and mottling on the neck and chest than do adults. The back of the neck and back are grey overall with a brown tinge due to the upper wing feathers are tipped in chestnut. Legs are olive grey, tending to pink close to the body. Feathered thighs are light, grey to white. Juvenile white birds are off-white with buff grey legs. Yearlings more resemble adults but with small white crowns, shorter plumes, and remnants of chestnut tipped wing feathers (Baker pers. comm.).
Chick: Dark form chicks at hatching are covered with a pale grey down, especially exuberant on the crown. Legs and bill are pink. White form herons have white down.
Voice: The harsh, deep, hoarse “Frawnk” call is the alarm, disturbance, taking flight, and aggressive call (Bayer 1984). This characteristic call is highly variable, ranging from a simple “frawnk”, “frank”, or “braak” when taking flight after disturbance to an aggressive series – “fraawnk, fraawnk, fraawnk, taaauw, taaauw”. The “Ark” call is given at the nest and part of the Greeting Ceremony. “Gooo” call is a bleat given at the end of a Forward display, in pursuit flights, and during aggressive encounters. “Roh” call, rendered “roh, roh, roh” is used to advertise territory either while Standing in conjunction with Arched Neck or in Circle Flight displays. As a feeding territorial call, it is answered in kind by birds on neighboring territories. It is also given when in active pursuit of another heron. A similar call, the Landing Call, is given at the nesting colony. “Go” call is a series of clucks, “go, go, go” given at the nest and approached. “Ee” call is a vocalization that serves as a social contact vocalization, given on arrival or departing. Birds also use a Bill Snap especially in defending a nest site and in other situations (Mock 1979). Bill Clappering is common among pairs.
Weight and mesurements: Length: 97-137cm. Weight: 2,200-2,500 g. It is the largest heron in North America, which lacks a representative of the giant heron group.
Field characters
The Great Blue Heron is identified by its large size, white crown, grey back and upper wings with black epaulettes, and chestnut thighs. It generally is seen alone, standing in an erect posture. When taking off and flying short distances, the Great Blue Heron often keeps its neck extended, but in level flight coils it back in typical heron fashion. Its wing beats are slow, powerful and steady; it can soar to height before gliding downward again. The bicolored dark and light grey wings with white spots on the leading edge are diagnostic.
It is distinguished from other dark herons in its range (Little Blue Heron, Tricolored Heron) by its much larger size. It is distinguished from the Sandhill Crane by its black (not red) head, lighter (not black) bill, and in flight by having its head pulled back (not extended).
The dark form Great Blue Heron is similar to the other large Ardea, whose wanderings can bring individuals into the other wise allopatric range of the Great Blue Heron. It is distinguished from the Cocoi Heron by having a white crown with a black crest (not black crown), grey (not white) neck with complex vertical neck stripping (not a single black line), light grey (not black) belly, chestnut (not white) thighs, and chestnut edges to the “shoulder” patches (not black epaulettes). It is distinguished from the Grey Heron by being larger (10-40%), having chestnut edges (not black) epaulettes, and chestnut (not grey) thighs. Distinguishing the field characteristics of juveniles is discussed in the Grey Heron account.
The white form of the Great Blue is identified by its size, its large, thick, straight bill, leg color, and plumes. It is distinguished from the Great Egret by its large size, cream yellow to buff grey (not black) legs, relatively larger, thick and straight (not thinner and slightly down curved) bill, presence of head plumes, less developed back plumes. It is distinguished from the medium sized white herons (Snowy Egret, immature Little Blue Heron, white phase Reddish Egret) by its much larger size and leg and bill color.
Systematics
Subspeciation of Great Blue Heron in North America, proper, is not as clear as one would hope. There is certainly clinal variation from north to south and east to west. However, clinal gradation, surprisingly, is not well studied due to the lack of specimens (Dickerman 1992). The number of subspecies described form North America range from seven to two (Oberhouser 1912, Payne 1979). Besides those recognized here, lessoni has been described as darker birds breeding in Mexico and repens a dimorphic form from coastal Venezuela described as light birds having short plumes and being smaller than occidentalis. These populations deserve closer scrutiny. The most interesting aspects of the Great Blue Heron’s systematics are the situations at the edges of its range, in the Caribbean and south Florida (occidentalis and by extension the similar dimorphic birds of the Venezuela coast) and in the Pacific north west of North America (fannini).
Occidentalis was erected by John James Audubon for the white birds of the Florida Keys. But these birds pair with dark birds and the subspecies name is now considered to apply to all the dark, white, and intermediate birds of south Florida and the Caribbean. Birds in this population are relatively larger than other populations, although likely this represents the end of a clinal variation in size down the Florida peninsula that remains to be quantitatively documented. For some time it has been known that there is a tendency for assortive pairing, by color, among Florida Bay Great Blue Herons. The most likely explanation for this situation is that the white birds evolved as part of a dimorphic coastal population in the Caribbean and this population is now in secondary contact in Florida with birds derived from the continental population (wardi). This interpretation is supported by recent behavioral and molecular studies by Heather McGuire (pers. comm.). Based on several criteria (morphological, white-dark pairing, molecular), the geographic variation shown in the southern Florida and Caribbean population is recognizable as subspecific but not species level of divergence.
The situation in fannini is similarly intriguing. This is a fairly isolated population of darker, shorter billed birds possibly with differences in breeding soft part coloration. This population may have diverged further from the eastern stock than is now appreciated (Butler pers. comm.).
Range and status
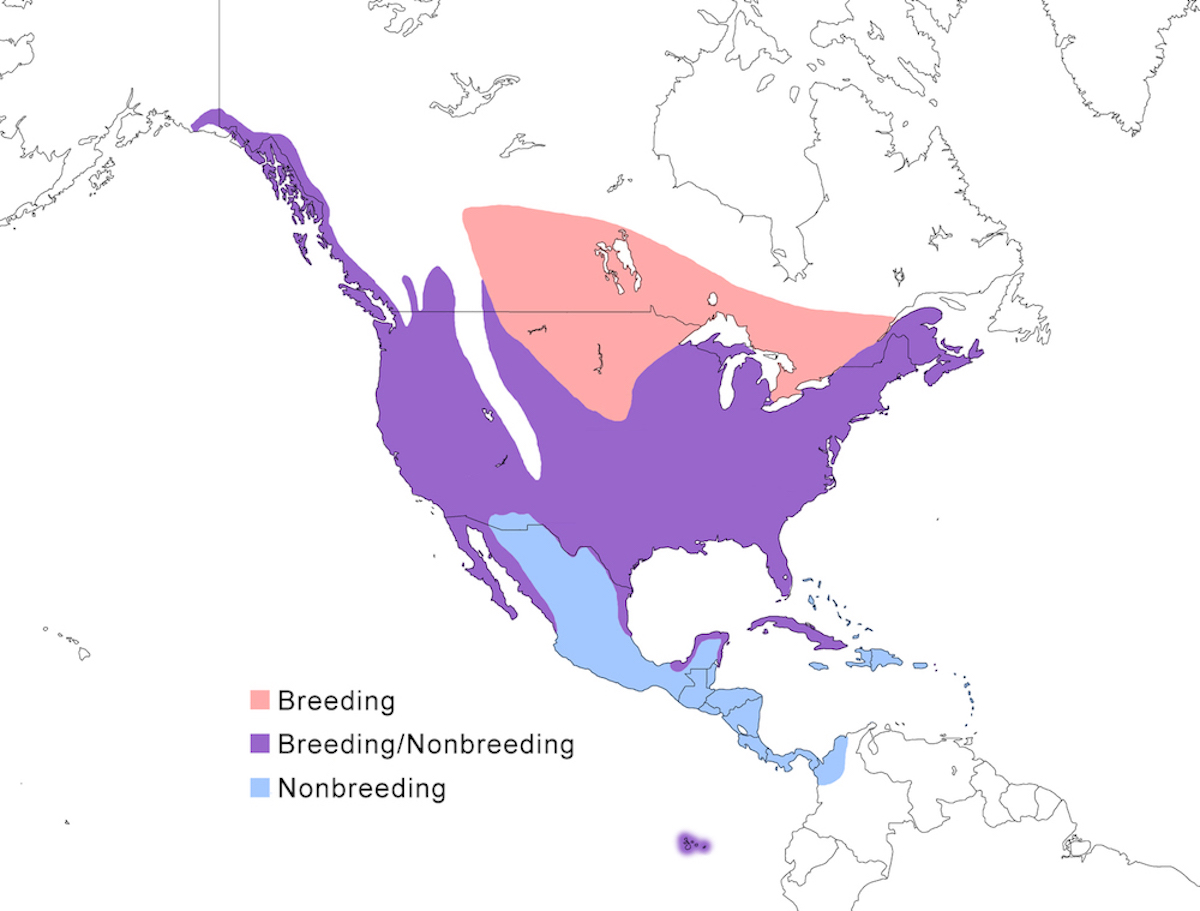
The Great Blue Heron breeds throughout much of North American except for high mountains and deserts, in Central America, on certain islands in the Caribbean and Pacific.
Breeding range: Its North American breeding range is from south east Alaska (Webster 1988, Butler 1997), British Columbia, across Canada from north Alberta, to central Manitoba, through south Ontario, south Quebec, New Brunswick, and Nova Scotia southward through the United States except the Rocky Mountains, into Mexico to Baja California (Palacios and Mellink 1983, Becerril and Carmona 1997), Sonora and Sinaloa on the west coast, and in Tamaulipas, Tabasco, and Yucatan on the east, also Cuba, St. Johns Virgin Islands, Los Rocas Venezuela, and Galapagos. Breeding status in Central Mexico is unclear.
The race herodias occurs over most of this North American range. The eastern race wardi occurs from Kansas and Oklahoma across the Mississippi river basin to Florida. The western race fannini occurs along the Pacific coast of North America from southeastern Alaska to coastal Washington. The Caribbean race occidentalis occurs in extreme south Florida, from Tampa Bay south especially in Florida Bay, and on Cuba, St. Thomas, and Los Roques, Yucatan (Lopez-Ornat and Ramo 1992). It occurs along the coast of Yucatan and Belize (Haas 1983) but has not been confirmed to be breeding there. It is also possible that it breeds in Venezuela (Morales pers. comm.). The race cognata occurs in the Galapagos Islands.
Nonbreeding range: Great Blue Herons have the largest nonbreeding range of any North American heron (Mikuska et al. 1998). Many remain rather far north in the nonbreeding season, from south east Alaska, through its USA breeding range except in the north central prairies. Birds remain widely dispersed in winter, but important wintering areas include Strait of Georgia, Puget Sound central California, the USA midwest, Colorado River, Great Salt Lake, Gulf of Mexico and Pacific coast of Mexico, and Florida (Root 1988, Mikuska et al. 1998). Great Blue Herons occur year round but with no evidence of breeding in the Bahamas. Nonbreeding range includes the Greater Antilles, especially Hispaniola and Cuba, with fewer in the Lesser Antilles south to Trinidad. It is also year-round in the Netherlands Antilles off the coast of Venezuela (Aruba, Bonaire). It is widely dispersed in northern winter in coastal Mexico to Guerrero on the west, Central America, rarely to Panama and northern South America, but occurring as far south as the upper Cauca Valley in Colombia. It occurs rarely in northern Venezuela and even more rarely southward - Great Blue Herons have been observed as far south as Rio Negro, Brazil (03 00 S) (Parkes 1998).
Migration: Migratory tendency depends on location. Herons migrate south in September and October, in numbers of a few to 100, flying in both day and night. Great Blue Herons from north central North America all are highly migratory in that their feeding habitats ice over. Eastern herons are more flexible, some shift southward, others remain rather far north, more in mild winters (George and Moore 1990). Fannini is non-migratory (Butler pres. comm.). There is no evidence for migration in occidentalis, although wandering occurs. Upon return migration, birds arrive early, usually in February to March (Butler 1992).
Great Blue Herons disperse widely after nesting. Dispersal records include Newfoundland, Greenland, Hawaii, Bermuda, ships in the North Atlantic (on which they have arrived in Europe), near the Azores, Canary Islands, and France (Casement 1995, Herroelen 1995, Dobson 1996, Guermeur 1996, Clarke 1999). Wardi disperse northward (Dickerman 1992). Birds of the white form have wandered as far north as Pennsylvania.
Status: Population overall appears to be at least stable over most of its range, increasing in eastern and southern North America, with some concern for a possible slow decline in the north Pacific population due to habitat losses and eagle predation (Butler 1997, Butler et al. 2000, pers. comm.). It is common to abundant throughout the rest of its breeding range to Mexico. It is rarer in the remainder of its breeding range, where in most cases its accurate status remains to be determined.
Population sizes and quantitative trends are not possible to determine accurately, because nesting sites are so numerous and highly dispersed (Butler et al. 2000). Available information includes over 35,000 birds along the south and east North American coast in the 1970’s, but the nesting population in Louisiana alone now numbers over 10,000 birds and has been increasing (Fluery and Sherry 1995). Few data are available from its inland range; although over 12,000 pairs are reported in Quebec, 13,000 pairs in Ontario, 5,000 pairs in Illinois, so numbers inland are not inconsequential. Overall the population has to number in the 100,000 to 250,000 range. The white morph in south Florida is thought to have been hugely reduced by plume hunting in the early part of the twentieth century but has recovered to about 1,500 individuals (Powell et al. 1989).
Habitat
The Great Blue Heron is a generalist in its habitat selection. Its long legs and large size allow it to feed from deep water to dry land. It uses freshwater and salt marshes, freshwater and mangrove swamps, estuaries, meadows, flooded agricultural fields and pastures, ditches, island shores, river banks, lake edges. It can feed on dry land when profitable. In salt water, they feed in coastal lagoons, mangroves, muddy and rocky seashores, tidal flats, sea grass flats, salt marshes and also in the surf and along beaches. The white form is found almost entirely in marine habitats, particularly tidal grass flats. In the nonbreeding season, outside the continental USA, birds tend to be marine using tidal flats, rocky shores, brackish lagoons and mangrove forests (Morales 2000). Overall, natural habitats tend to be more important than man made ones (Heitmeyer 1986). It requires trees or bushes to nest in most locations. Although a bird of the low lands, it has been recorded up to 2,600 m in South America. These herons are flexible, and can respond to changes in habitat availability (e.g., Elphick and Oring 1998).
Foraging
This heron feeds usually in the water or at its edge. Over 90% of the time, it feeds by Standing or Walking Slowly. It also uses active behaviors such as Hovering, Plunging, Jumping, and Swimming Feeding to forage in even deeper water than its substantial leg length would otherwise allow. It uses Standing Flycatching, Probing, Pecking, Running, Hopping, and Wing Flicking. Given the advantage of their size, they occasionally rob other birds of prey (e.g., Bildstein1980, Squires 1998, Merrifield 1992, Bayer 1985a, Squires 1998). Similarly, being obvious and not so agile as smaller birds, they are subject to piracy from other species (Johnson et al. 1996).
Prey taken differ with different behaviors. Forbes (1987a) found that by Standing birds caught more active prey; whereas by Walking, birds caught more sedentary prey; by Feet First Diving, birds took dead and floating prey. Net return was lowest for Standing, higher for Walking and highest for Feet First Diving. So Great Blue Herons choose foraging behavior on the basis of its profitability, unusual or energy costly behaviors being used when the return is high (e.g., Morey and Smits 1987). Given their flexibility, Great Blues can take advantage of unusual foraging opportunities. For example, herons Dip behind fishing boats as nets were hauled (Ewins and Hennessey 1992), appear at grunnion runs (a beach spawning fish) (Griem and Martin 1997) or catch fish attracted to floating bread (Zickefoose and Davis 1998).
Herons take advantage of food provided by aquaculture, such as pond raised catfish, crawfish and bait fish, and are considered to be significant nuisances to these industries. Local Great Blue Heron populations can obtain up to 44% of their diet from catfish ponds and up to 50% from trout hatcheries (Glahn et al. 1999a, b). However, in a controlled experiment no difference in stock was found between aquaculture facilities in which herons foraged and control situations (Glahn et al. 1999a, b). Herons were found to be poor predators on healthy fish but good predators on sick fish (Glahn et al. 2000). Unhealthy fish and less desirable species, such as sunfish (Lepomis), were the preferred prey of Great Blues at catfish ponds.
Great Blue Herons feed by day or night. Especially in tidal situations, herons feed in relation to the tidal cycle, feeding at night at the proper tide (Bayer and McMahon 1991, Austin 1996). Great Blues also feed at night in nontidal conditions, so nocturnality is a typical part of their behavioral pattern and they are well adapted for night vision (McNeil et al. 1993, Rojas et al. 1999).
These herons typically defend their feeding sites, either a large territory held alone or individual areas when feeding in an aggregation. Territories are defended in both winter and summer. In Oregon, territories averaged 8.4 ha on the coast and 0.6 ha inland; In Canada, males defended territories while females and juveniles fed communally (Butler 1992). Pursuit and Supplanting Flights are common, as are standing displays such as Upright displays. They will advertise their territorial claim by their presence with Roh call and also by Arched Neck and Circle Flight displays (Bayer 1985b). Their most interesting display is given on the feeding grounds, the Upright and Spread Wing display: two herons encounter each other wings spread and drooped, with neck extended, head high over the back. The bill becomes more vertical and the back of the head further on the back as the display continues. Herons also use Forward and Vertical Displays on the feeding site, the former merging into duels, which can be sufficiently severe that herons are injured.
The Great Blue Heron, being a large bird, can catch and use both small and large prey. Handling time depends on the size and the defenses of the prey item. Herons tend to grab prey but can spear them as well (George 1941). They attack surprisingly large prey, but large and defensive prey can overwhelm herons, even causing their death (Wolf and Jones 1989). For a bird that seldom catches a prey item, increased handling time seems not to be a great constraint and Great Blue Herons devote considerable time to subdue and prepare prey for swallowing (Forbes 1982, 1989). Herons can readily loose their relatively large prey to other birds (e.g., Squires 1998). Their foraging technique takes time and experience to learn. Juveniles are less effective than adults, and their skills increase with age (Quinney and Smith 1980).
The Great Blue Heron typically eats fish, generally relatively large fish such as Micropterus, Lepomis, and Esox. But overall prey items include a wide range of insects, fishes, amphibians, birds, mammals and reptiles. Birds are often reported; and in the west, mammals are frequently taken. In fact, seemingly atypical prey can in certain places be important in a heron’s diet, such as is the case for hatching sea turtles (Sage 1994), ground squirrels (Hoffmann 1997), voles (Collazo 1985), and birds (Chapman and Forbes 1984, Vaniman and Luna 1998). Herons, especially on islands such as Florida Keys and the Galapagos, frequent human habitations for scraps of food provided for them, and these may become important dietary supplements.
Breeding
The Great Blue Heron is generally the first heron to begin nesting, due to its long nest period. Nesting in most of its range including continental USA, Cuba and Mexico, begins in February through April continuing to June through August. In extreme southern Florida nesting is nearly year round, more concentrated in winter, December–April.
As a generality, Great Blue Herons need tall trees with some isolation from human disturbance and nearby aquatic feeding areas. These areas may be singular sites that the heron can use continually as food is renewed or are a series of sites that can be used sequentially during the nesting season. Colonies tend to be well dispersed, but that are located so that the birds can fly 3 to 24 km to surrounding wetland complexes (Gibbs and Kinkel 1997, Dowd and Flake 1984, 1985). Colony size is correlated to the amount of wetlands within flying distance from the colony site (Gibbs et al. 1987, Gibbs 1991).
Great Blue Herons tend to nest in relatively undisturbed sites, buffered from disturbance, with low road density, and surrounded by large forest stands (Scharf 1989, Gibbs and Kinkel 1997). Colony sites tend to shift around, sometimes for no apparent reason but at others due to tree mortality (Julin 1986, Dusi and Dusi 1996). They are flexible, such as in their rapidly occupying re-established beaver ponds (Potter and Barkley 1997).
Great Blue Herons nest either solitarily or in colonies. Colony size can vary from year to year (Julin 1986). Regionally they tend to nest at a few large and many smaller colonies, the larger sites being more stable (Iverson 1993, Castrale 1994). Nests are stick platforms that vary from 0.5 to over 1 m across. Nests that survive the winter are rebuilt and enhanced, becoming larger over several years (Gretch 1989). Surviving nests also serve as display sites and if unclaimed by herons can be used by other species such as owls (Burkholder and Smith 1988). Nests tend to be put in the taller trees of those available (Carlson 1995). They do use bushes where these are more available, especially mangrove bushes in the tropics. They also nest on artificial structures (Henny and Kurtz 1978, Paton and Kneedy 1993), on the ground in protected sites, or on cliffs.
The courtship behavior of the Great Blue Heron has been well studied for decades (Cottrille and Cottrille 1958, Meyerriecks 1960, Mock 1976a). Herons change mates and nest sties from year to year (Simpson et al. 1987) and so prey selection is a critical part of the annual cycle. Birds sometimes gather on foraging grounds to display. At a colony site, a displaying male usually first secures a display site, often an old nest site. During pair formation, males defend the nest and site with Forward displays, jabbing, and supplanting flights.
An Advertising Call is seldom given, the bird instead advertising with a highly stereotyped Stretch display, which includes much lateral swaying. The Snap display includes an audible mandible 'clack'. Other displays used include Wing Preen, Circle Flight, Twig Shake, and Fluffed Neck. Crest Raising is performed by both sexes throughout the breeding season. Aggressive displays include the Upright, Arched Neck and Forward. These are not used in predictable ways, and there is much individual variation in their use. After pairing, Contact and Non-contact Bill Clappering are common.
Eggs are pale blue, having a size range of 61.3-65.6 x 41.9-46.5 mm (Schonwetter 1967). Eggs are laid at 2 to 3 day intervals. The clutch size varies from 2 to 7 eggs, increasing from south to north. Incubation takes about 25-29 days, most typically 28 days. Incubation is continuous after the first egg, consuming 54 minutes of each hour (Pratt 1970). Nest relief occurs once per day. Nests are seldom left unattended during incubation. Males tend to stay at the nest during the day, and females at night.
Both parents attend the nest and young, relieving each other 2 to 5 times per day. Hatchlings are not left alone until after 28 days old (Pratt 1970). Feeding activity peaks when chicks are about 4 weeks. Both parents feed the young by regurgitation, as many as 6 to 10 times per day. Nesting success depends on food supplies, generally producing two to three young per successful nest. Chicks fledge at 60 days and leave the nest from 64-91 days. (Pratt 1970).
Mortality of chicks is often high, one to two usually being fledged. Among the highest success rates reported was about 3 per nest in Nova Scotia (Quinney 1983). Colony characteristics, food supplies, parental care and predation affect nesting success. Herons in smaller colonies were more likely to fail completely but also more likely to raise three young than herons in larger colonies, overall there being no difference in fledging expectation (Butler et al. 1995). Nesting success is limited by food supplies and by the ability of adults to supply sufficient food (Quinney 1982, David and Berrill 1987, Mock 1985, 1987, Sullivan 1988, Bennett et al. 1995). Chicks in competition for food can attack other chicks (David and Berrill 1986, Mock et al. 1987), but this trait is not as pronounced as in other species and chicks killing other chicks is not common. Becoming increasingly appreciated is the extent to which predators such as crows (Corvis), eagles (Haliaeetus), vultures (Cathartes), owls (Bubo), wolves (Canis), raccoons (Procyon) and bears (Ursus) attack nestlings (Gretch 1987, Norman et al. 1989, Robinson et al.1991, Butler 1992). Heavy predation can affect nesting success (Seabolt 1984), and in some parts of the range, the populations of these predators are increasing.
Population dynamics
The population dynamics of the Great Blue Heron are generally understood due to a long term banding record and intensive studies at colonies (Henny and Bethers 1971, Henny 1072, Blus et al. 1980, Blus and Henny 1981, Crawford and Neel 1997, Butler 1997). However no single colony or group of related colony sites have been subjected to long-term study of marked birds. They appear to begin to nest at two years, although younger birds do attempt nesting. Mortality rates are high in juveniles. In the first year they are 69-71%, decreasing thereafter and with regional differences (Henny 1972, Bayer 1981). Highest postfledging death rates are from August to December (Owen 1959), probably related to the difficulty of learning to feed (Quinney and Smith 1980). Considerable study has investigated contaminant burdens in Great Blue Herons, but there is no evidence of population impairment (e.g., Henny and Bethers 1971, Blus et al. 1980, 1985). Mortality on the wintering grounds may be important (Blus and Henny 1981), particularly the mortality occurring during severe winters of birds wintering far north. Overall, the Great Blue Heron is a relatively long-lived bird (Crawford and Neel 1997).
Being so large, the adult Great Blue Heron is immune to most predators. However they are harassed and their prey taken by eagles and it is likely that predation by eagles is more important than previously appreciated (Forbes 1987b). Great Blue Herons can kill each other in territorial disputes (Nehls 1992).
Conservation
This is a very adaptable species. Overall populations are stable or increasing, so there is no range-wide conservation concern. Certain coastal populations occupy relatively small areas. These needs particular conservation attention. Colony site protection is the most critical conservation issue for Great Blue Herons. Range-wide, nesting sites are not limiting, but the inexorable loss of potential sites, particularly those most efficiently positioned near productive feeding areas, is continuing, Herons adversely after nesting trees and feeding conditions change from time to time. As a result heron colonies naturally shift sites, particularly the smaller ones. So colonies need to be protected and managed on a landscape scale to preserve a matrix of potential sites. Research is clear that nesting Great Blue Herons prefer to avoid human disturbance. They nest away from disturbance and shift colony sites and breeding dispersion relative to disturbance factors (Scharf 1989. Gibbs and Kinkel 1997). In Nova Scotia human disturbance affects nesting success (Quinney 1983), probably also the case elsewhere. Colony sites need to be protected from disturbance, ranging from habitat destruction to intrusion by humans or their domestic pets. Buffer zones are required around active colony sites (Rodgers and Smith 1995b, Carlson and Mclean 1996). Colony site protection effort needs to be active, ongoing, flexible, and at a landscape scale.
The adaptability of the species can put it in conflict with human activities. Great Blue Herons are able to take advantage of abundant food supplies made available by aquaculture operations such as trout hatcheries, catfish ponds, and bait fish (Parkhurst et al. 1992, Hoy 1994, Stickley et al. 1995, Pitt and Conover 1996, Pitt et al. 1998). Studies have shown that, except in restricted situations, depredation caused by this species is not economically important, and where it is important it can be managed without harming the birds (Glahn et al. 1999a, 2000, 2002). Many birds are killed legally and illegally in the USA at aquaculture facilities. Ove 12,000 herons were reported to be killed legally from 1987 to 1995 in southeast USA (Belant et al. 2000). Such killing of a long-lived species can be expected to affect population viability. There is little valid justification for killing Great Blue Herons because their purported impact on aquaculture. In Louisiana, populations have increased due to the food man available by crawfish aquaculture (Fluery and Sherry 1995). In Florida Bay, white herons facing low reproduction attributable to declining environmental conditions affecting food supply are being maintained by taking handouts from people (Powell and Powell 1986). Being a top predator, Great Blue Herons accumulate persistent toxins, and many studies have been conducted of contaminant loads and possible effects. So far, population effects have not been shown and individual effects are subtle. It is possible that the versatility of the species includes its ability to deal with contaminants. As a result, this species can serve as an environmental sentinel, and monitoring contaminant loads should continue range wide. Great Blue Herons are impacted by catastrophic toxic events, such as occurred at the Salton Sea, USA. Management of such incidents is critical in that they can affect wintering birds concentrating in such areas.
Conservation of feeding habitat and food supplies is another critical issue. Throughout its range, natural situations are preferred over altered habitats. Conservation of the wetlands, rivers, streams, and other aquatic habitats supporting this species is essential. This is best done as part of landscape-scale wetland conservation programmes. In many areas the Great Blue Heron, as one the larger and more apparent water birds, can be used as a ‘poster' species to encourage local and regional wetland and stream conservation action. Despite being continent wide in distribution, the species is principally a coastal bird in the winter. Conservation of coastal habitats in the southern USA, Mexico, and the Caribbean is essential.
Research needs
This is a well-studied species. Much of its basic biology is known and it has been used as a study model for behavioural and evolutionary questions. Infraspecies variation needs to be better understood, not only the validity and utility of described subspecies but also clinal and other patterns of geographic variation. Biochemical studies of these relationships are needed, especially in the Caribbean and Central America. It is possible that these forms represent a significantly divergent lineage. Nesting datus of the species in Central Mexico, coastal Mexico and Belize, Central America, and the Caribbean (Bahamas especially Andros Island, Cuba, Lesser Antilles, and the islands off Venezuela) needs to be clarified. Migratory movements and wintering areas of various subpopulation need to be better understood. Colour marking, satellite telemetry, and biochemical studies can accomplish this. Because its nesting is highly dispersed over its North American range, the overall and seasonal population size of this species is poorly known and its trend is only guessed at. It is important to develop a regional and range-wide census and monitoring programme for this species, giving due consideration to the error bias inherent in such counts (Gibbs et al. 1988, Dodd and Murphy 1995). Studies need to continue of the relationship of herons to aquaculture focusing on the populational risks of killing birds and ways to mitigate adverse impacts of the birds to aquaculture.
Overview
The Great Blue Heron is the North American representative of the large heron group. It is a specialist at patient feeding, tending to catch a few large prey items each day, seemingly being pressed for time only when feeding young. That success varies with age has important demographic implications, and may explain why maturation is delayed to a time when the birds have mastered the species’ difficult feeding tactics. Birds tend to choose to forage in profitable sites, changing locations as needed. Birds from a single colony can feed in different situations (Parsons and McColpin 1995). Despite breeding in colonies, birds show a mixed social strategy tending to occupy and defend individual feeding areas. Although some colony sites are occupied for many years, it is more common for colony sites to shift slowly as the breeding habitat degrades or available food supplies shift over the long term. This variability in nesting site use allows the species to adapt to ever-changing conditions. Taking advantage of artificial food supplies can increase nesting success and population size. It is flexible in feeding timing, especially along the coast (Horvath and Moholt 1986, Austin 1995). So this adaptable species has an admirable ability to exploit available habitats and food resources. As a result it is abundant, widespread, and well-known throughout its extensive range.