Intermediate Egret
Ardea intermedia (Wagler)
Ardea intermedia Wagler, 1829. Ibis, vi, p. 659: Java.
Subspecies: Ardea intermedia plumifera (Gould), 1847: New South Wales; Ardea intermedia brachyrhyncha (Brehm), 1858: Blue Nile, eastern Sudan.
Other names: Yellow-billed Egret, Plumed Egret, Median Egret, Smaller Egret, Lesser Egret in English; Garceta Intermedia in Spanish; Aigrette intermediare in French; Mittelreiher in German; Middelste Zilverreiger in Dutch; Средняя белая цапля in Russian; Kuntul perak in Indonesian; agak, Talabong in Pilipino; Chu-sagi in Japanese; Zhong bailu in Chinese.
Description
The Intermediate Egret is a medium white heron with a relatively short yellow or bicolored bill and, in breeding, exceptionally long back plumes.
Adult: The plumage of this heron is all white with cream or off white tinge. The bill color is basically yellow, but varies seasonally and geographically from yellow, black, to yellow and black, to yellow and brown. The irises are yellow and the lores are pale yellow. The legs are dark, sometimes two toned. In breeding, a train of long aigrette plumes extends along the back to 10 cm beyond the tail. Similar plumes extend from the lower neck forming a pronounced bib. Head has a slight crest but no plumes. Soft parts change color in breeding season, in geographically variable ways (see below). Coloration changes rapidly during nesting, being intense during courtship and fading thereafter.
Variation: The sexes are alike. Geographic variation in size and soft part color is recognized in three subspecies.
Intermedia is intermediate in size among the subspecies. Its bill is yellow, often with black tip. Legs are slate grey, dark green or brown. Feet are grey to black. During courtship, lores are yellow green, bill is black, and legs are black. After courtship in breeding, bill turns yellow below remaining black above.
Plumifera is smaller. Its bill is yellow orange to buff. Upper legs are variable, pale brown, green, grey, or yellow. Rest of lower legs and feet are dark brown. Lores are green yellow to yellow. The irises are horn. During courtship, the bill is deep pink to red with a yellow tip. Base of the lower bill is green, lores bright green, and irises are red. Upper leg is ruby red, lower leg is variably ruby with or without black on the front, or black with red wash. Post courtship, during incubation and chick rearing, the bill becomes dull red changing to orange yellow and the lores become pale green changing to yellow green to yellow. The irises turn yellow. Upper legs become yellow and the lower leg, grey.
Brachyrhyncha is larger. Its bill is all yellow or yellow orange. Upper legs are yellow. Lower legs and feet are dark brown to black. During breeding, the bill is pink red. In courtship, lores turn bright green, irises red, and upper leg pink red. The lower legs are black with a yellow line along the side (the line tuning pink red in courtship), and the feet are black.
Juvenile: Juveniles look like adults but may have a faint yellow wash to plumage.
Chick: The chick is covered in white down with white crest. Its soft part colors change with development (McKilligan 1990a). The bill color is variable with time and among individuals. Generally it starts yellow, turns brown near the head, and becomes mostly brown in time. In a study of plumifera in Australia (Maddock 1988, 1989b), chicks were found with several types of bill color: beak all yellow (37%); black-tipped yellow (18%); variegated black and yellow (45%); and an occasional all black beak. The mouth color was also variable. Eighty percent of mouths (hard palates) were all black, 2% were black in front and yellow behind, 1% all yellow and 2% pink and black. The lores are bright yellow or yellow and black and the irises are yellow white turning to pale yellow. Legs are olive green turning pale green then grey green on back black on front.
Voice: This egret is normally fairly silent, Most vocalizations are quiet, and its soft buzzing call is unique among herons. Soft repeated “Glock” call, rendered “glock, glock”, and harsh “Kroo” call, rendered “ kroo, kroo”, are alarm calls (heard in Australia but not Africa – Marchant and Higgins 1990, Blaker 1969). The “Kraa” call, rendered “kraa, krr”, is a flight call. The “Kroh” call, rendered “kroh, kroh”, is a greeting call. The “Grrk” call, rendered “grrrk, grrrk”, is a soft, two-syllable, rasping, staccato sound used in Greeting Ceremony (called a soft buzzing call by Blaker 1969). Chicks beg with “chi, chi, chi”, which becomes “khe, khe, khe” or “cro, cro, cro” with age.
Weights and measurements: Length: 65-72 cm. Weight: 400-500 g.
Field characters
The Intermediate Egret is identified during the breeding season by its extensive plumes, especially the very long back plumes and also by a lack of head plumes. It is sleek bird, with a bill relatively short for a medium aquatic heron. It has a long head and neck, often held in an s shape when standing or flying (McCanch and McCanch 1991), but relatively shorter than that of the Great Egret. In flight it resembles that of the Great White Egret, rather buoyant and with slow wing beats. Races are variably identifiable by courtship and breeding bill and leg color.
In the nonbreeding season, it can be easily confused with other white herons. It is distinguished from the Eastern Great Egret in Asia and Great Egret in Africa by smaller size, by its relatively shorter, deeper bill, shorter neck (relative to the exceptionally long necked great white egrets), bicolor (not all black) legs and the thin line of the mouth gape ending at the eye rather than continuing past the eye. It is distinguished from the Little Egret by its somewhat larger size, yellow to bicolor bill, yellow lores, black feet (not yellow), usually not all black legs, back and neck plumes and lack of head plumes in the breeding season, and less active feeding behavior. It is distinguished from the Eastern Reef-Heron by its slightly larger size, yellow or yellow and black bill (not horn upper), bicolor to dark legs and black (not yellow) feet, taller and more graceful less haunched appearance, and by its habitat choice (inland and protected coastal sites as opposed to open reef crests). It is distinguished from the Cattle Egret by its larger size, larger bill, relatively longer neck, dark (not yellow) legs, and slim appearance.
Systematics
The generic position of this species has long been a puzzle. Often assigned a genus intermediate between Ardea and Egretta (Casmerodius, Mesophoyx) or to Egretta due its extravagant plumes, it has been shown to be closer genetically to Ardea. The disjunct, continent-based distribution of the recognized subspecies and confusing individual and geographic variability in soft part color suggest that the taxonomic relationship among African, Australian and Asian forms should be re-examined. There may well be more than a single species within this cluster of medium white plumaged herons.
Range and status
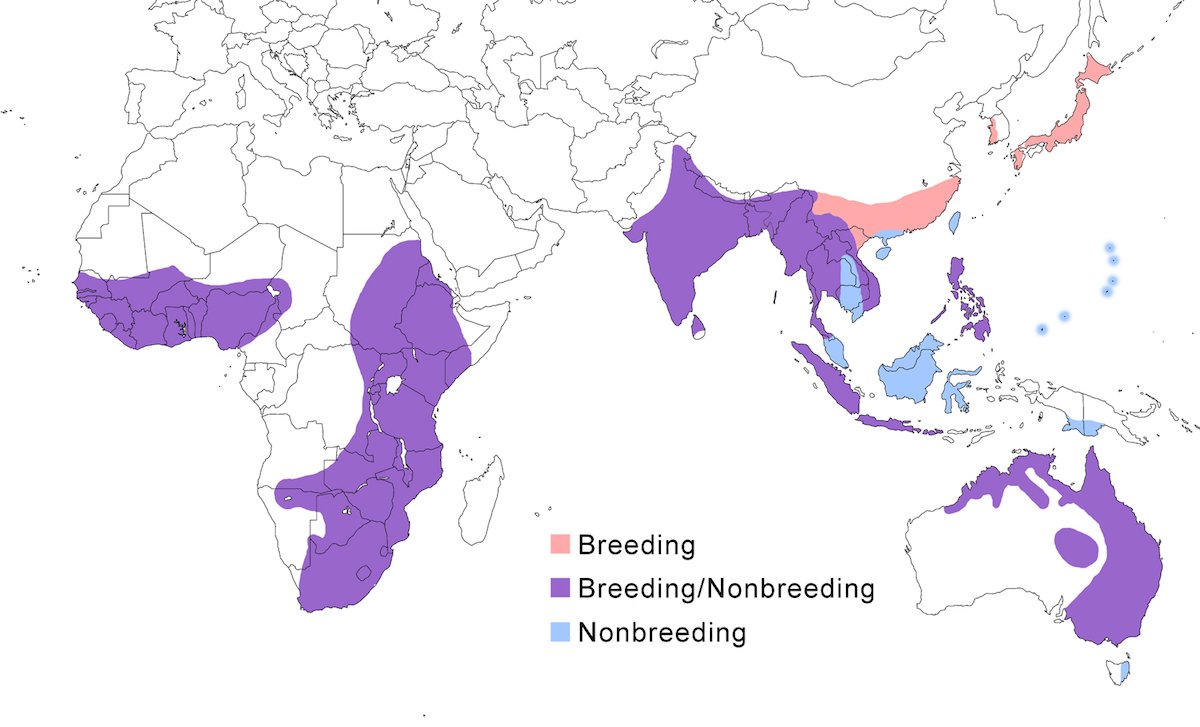
This species occurs in apparently isolated populations in Africa, southeast and east Asia, and Australia.
Breeding range: In Africa, brachyrhyncha occurs south of the Sahara, its distribution avoiding deserts and jungle. In west Africa, it is in Senegal, Mali (Wallace et al. 1992), Guinea-Bissau, Nigeria and Chad but is absent from desert and equatorial rain forest. In west Africa it occurs in north Sudan, north west Ethiopia, (avoiding the desert horn of Africa), Uganda, Kenya, Botswana, Tanzania, Zimbabwe, Zambia. In southern Africa, it occurs in north central Namibia to South Africa.
In Asia, intermedia breeds in Pakistan, India, Nepal, Sri Lanka, China (north to Szechwan, Hunan), Korea, Japan (Honshu, Shikoku), and Indonesia (Java, Sumatra).
In Australia plumifera occurs in northeast Western Australia, west to Queensland, south to Victoria, with large breeding colonies throughout the Murray-Darling inland river system.
Nonbreeding range: North Asia populations spend the nonbreeding season in the Malaysia, Philippines, and Indonesia (Borneo, Sulawesi). Australian birds occur in south New Guinea, Tasmania, and New Zealand.
Migration: North Asian birds undergo true migration, leaving Japan in September–October moving to and through peninsular and insular Malaysia and across the Luzan Strait to the Philippines and to Borneo (Sabah-Sheldon et al. 2001). Birds may winter in Oceania (Carolines, Marianas. There is clearly nonbreeding seasonal movement between breeding areas in north east Australia and nonbreeding areas in New Guinea (Geering et al. 1998). In India and Africa, the species has been generally considered residential, but in most areas it undertakes partially or complete population shifts related to wet/dry conditions. Post breeding, birds disperse and shift from wet season nesting areas to dry season nonbreeding sites. In droughts, birds concentrate around permanent water. It is semimigratory in Australia. Australian birds disperse and shift and some migrate to Tasmania, New Zealand and southern New Guinea, returning to their natal colonies during the breeding season (Geering et al. 1998).
Dispersal results in occurrences outside its normal breeding range, including Mauritania, Cameroon (Martinez et al. 1996), Cape Verde Islands, Jordan (Dead Sea), Hong Kong, Tasmania, New Zealand, Norfolk Island. Spring migrants overshoot, resulting in dispersal records occurring as far north as Russia (Ussuriland, Sakhalin Island), North Korea, and Midway Atoll (Richardson 1999).
Status: There are few available data on population sizes or trends, but the species is common to abundant in many places where it occurs and presumably it is stable in most areas. It is locally common to abundant in places throughout its range. Numbers in the hundreds to over a thousand have been recorded nesting in Kenya, Mali, Ethiopia, Zimbabwe, India, Pulau Dua Indonesia, and Australia. It has increased in numbers and range in west Africa (Turner 2000); a population estimate for Tanzania is fewer that 12,000 birds (Baker and Baker in prep.). It is abundant in nonbreeding areas in Malaysia, Philippines and Papua New Guinea (Lansdown et al. 2000, Maddock 2000). It is common in Bali. About 130,000 were counted in a regional survey in Papua New Guinea (Halse et al. 1996). Numbers in the hundreds of thousands Intermediate Egrets were also found in north Australia (Morton et al. 1993). However, in some areas the species has suffered declines. Once the most common egret in Japan, it has declined markedly since the 1960's. In east Australia decreases from year to year in colony size has caused concern (Maddock 2000), although fluctuations in colony numbers seems characteristic of the species.
Habitat
The Intermediate Egret uses a wide variety of flood plain wetlands but principally shallow water wetlands and pastures (Blaker 1969, Sato and Maruyama 1996, Tojo 1996, Takumi and Ezaki 1998). Habitats include ponds and bilabongs, fresh water marshes, swamp forests, water courses such as rivers and streams, fresh and alkaline lake margins, reservoirs, sewage ponds, rice fields, wet meadows, wet fields, and flooded and dry pasture. The less frequently used coastal habitats include mangrove swamps, salt marsh, intertidal flats, tidal streams and rivers. It very commonly uses wet meadows and grasslands near water and is not characteristically coastal. It prefers sheltered water less than 80 cm deep with emergent grasses. Egrets tend to avoid habitats where vegetation is too thick to feed in (Sato and Maruyama 1996).
Foraging
Intermediate Egrets are diurnal and typically solitary hunters. On occasion they do form small loose flocks or aggregations in the hundreds, In Africa it has been suggested that these are old reports and that flocks are not seen today (Baker and Baker in prep.). They hunt primarily by Standing and by Walking Slowly in shallow water or on damp ground in a slow and graceful manner (Recher and Holmes 1982, Recher et al. 1983, Sodhi and Khera 1986). When walking they can have a decided strut, that displays their neck plumes (Hancock 1999).
They also can feed in deeper water by walking on the matted vegetation. When Standing or Walking they use Gleaning, Head Tilting, and Peering Over. More active behaviors include Foot Stirring, Hopping, Hovering and Plunging (Klapste 1976, Sivasubramanian 1988). Head Swaying is used for feeding on insects in pasture. They have been reported to feed commensally, following spoonbills (Maddock 1992).
The diet of this heron is principally small fish in aquatic habitats and insects in terrestrial habitats, especially grasshoppers and crickets (Maddock 1986, Baxter and Fairweather 1989, Sodhi 1992). Fish include eels, perch (Macquaria), gudgeons (Eleotridae) and in Australia the introduced mosquitofish (Gambusia). It also eats frogs, snakes and lizards. Invertebrates include crayfish, leeches, water bugs, and dragonfly larvae in aquatic habitats and grasshoppers, mole crickets, bugs, beetles, spiders in drier habitats.
Breeding
The Intermediate Egret breeds typically in the wet season, beginning just past the height of the rains or floods. In Africa it may be at the waning of the long rains or the short rains, depending on location. As a result, the months of breeding are highly variable across the range. For example breeding has been reported in July–October in Senegal, March–August in Kenya, September–October in South Africa, July–September in north India, November–February in south India, February–July in west Java, December–February in east Java, November–April in Australia. Timing in an area may change among years, due to drought or floods (Geering 1993).
The Intermediate Egret is highly colonial, nesting in mixed species colonies of other herons, spoonbills, ibises, and cormorants, which may be very large. Pairs do nest singly as well. Colonies are typically sited on trees in and near water, in inland swamps or mangrove swamps. Trees used in Australia are eucalyptus, mangroves (Avicennia), paper bark (Melaleuca), and ironwood (Casuarina). They also nest lower to the ground, in bushes and reeds. Colonies may be used erratically, depending on water conditions
The nest is a shallow platform of loosely woven reeds, sticks or twigs 60-80 cm across and 20-40 cm deep. The nest is placed from 1.5-20 m above the water or waterlogged ground, but there is a tendency to nest high in the trees (Ando 1993). Both sexes build the nest, with the female usually completing it with material brought by the male. Both males and females steal sticks from nearby nests.
As might be expected, courtship makes ample use of the extravagant plumes of the species. Birds claim a display and nesting territory. Males occupy, advertise, and defend the site using Standing, Preening, Upright, Twig Shake, Forward, Supplanting Flights, and Flap Fights. In the Forward, the body is fluffed out and all plumes are erected.
Courtship displays are well described (Blaker 1969a, Marchant and Higgins 1990). Courtship displays reported are Stretch, Standing, Preening, Wing Preen, Head Toss, Snap, Back Biting, Bill Clappering, Flap Flights, and Circle Flights, which may have neck extended component. Stretch displays are rare. The predominant display is the Snap, in which the bird raises it back plumes and crest, flexes it legs and bobs up and down rapidly. In Africa the birds are reported to have their head and neck horizontal whereas in Australia they are reported to have the bill tucked to the chest (and the behavior there has been called Bobbing). There is no bill snap or twig grasping component or calls in either region. This display is most common at around the time of pair formation.
After pairing, the Snap continues to be used by both birds as the Greeting Ceremony in which plumes and crown feathers are raised, approached bird stands and does the Snap several times. Arriving bird may also give a Snap display and give the Kroo call or the quiet buzzing Grrk calls. Paired birds also spend considerable time at the nest site Standing, Preening, Wing Preen, Back Biting with Bill Clappering and intertwining necks. Exchanging sticks includes neck arching and flaring of plumes.
The eggs are pale green, smooth and slightly pitted. They average 47-49 x 35-40 mm. Eggs of the smallest race are 45 x 32 mm. The clutch is usually 2-3 eggs; range is 2-6. Incubation is by both sexes and takes 24-27 days.
Hatching is asynchronous. Adults brood for 12 days. Nests and young are attended by both parents who defend eggs and young from ravens and hawks (McKilligan 1991), crouching over nest, raising plumes, and pointing bill to predator (Marchant and Higgins 1990). The young are fed by regurgitation, first onto nest floor and later chicks take food from the parent’s mouth. Competition for food can occur among siblings (McKilligan 1990a).
Young are semialtricial. Pinfeathers appear at day 4. They can leave the nest at 24 days; but return for feeding. They fledge at about 40 days and leave the colony at about 70 days. Success reported as relatively high in Africa and in Australia, where survival of hatchlings to fledging was 96% and 88% of nest fledged at least one young (Maddock in Marchant and Higgins 1990). Nesting success is higher in wet than in dry years (Maddock and Baxter 1991, Baxter 1994).
Population dynamics
Birds in breeding plumage but not breeding coloration returned to natal colony in first year but did not nest (Maddock in Marchant and Higgins 1990). Survival has not been evaluated.
Conservation
This species is widespread over its range, locally abundant, and by all measures in an admirable conservation situation. As a bird that seasonally uses grasslands, it has benefited from the clearing of forests for agriculture and the extension of irrigation into dry areas. It uses human-altered situations, such as reservoirs, ponds, and rice fields. However, there are threats to these habitats. Changes to floodplain habitat on which it depends for nesting are caused by drainage, levee breaking and salt intrusion, grazing, and burning (Marchant and Higgins 1990, Maddock 2000). Changes in rice agriculture can affect the use of rice fields by the Intermediate Heron (Lane and Fujioka 1998). In coastal eastern Australia, there has been a significant decline in the breeding population in the period 1988 to 1998, exemplified by the 98% decrease shown by long-term monitoring at the Shortland colony (Maddock 2000), with no evidence since of a recovery (M. Maddock pers. comm.). Breeding at colonies in the inland Murray-Darling river system has been erratic, with long periods without nesting, such as in four of seven breeding seasons in the Macquarie Marshes 1990-91 to 1996-97 (Maddock 2000). The decline in Victoria, where its Conservation Classification is Critically Endangered (NRE 2000), has been especially severe, with no significant nesting event for about 20 years (O'Brien pers. comm. in Maddock 2000).
Despite its use of rural human environments, the Intermediate Egret is considered to be shier than other egrets and it is thought to be easily disturbed. Colony sites near potential disturbances need to be protected. Inland colonies, such as on regulated rivers in Australia, must continue to receive adequate water flow to ensure the long-term continuation of successful breeding (Maddock 2000). Close attention must be paid to the conservation of foraging and roosting habitats along migration routes.
Research needs
African, Asian, and Australian Intermediate Egrets appear to differ in size, breeding and courtship coloration, and courtship displays. This suggests that patterns of geographic variation and the taxonomic relationship among these forms should be re-examined, including by the use of molecular techniques. The rapid change in breeding colours makes these difficult to document, and a careful comparative study needs to be undertaken. It is possible that these populations might be found represent different species. Continuation of study of dispersal patterns in Australia and the initiation of similar studies in other parts of the range are needed to better understand seasonal movements and provide data in support of conservation and habitat management efforts.
Overview
The Intermediate Egret appears intermediate not only in size, between large and small egrets, but also appears to be intermediate in its ecology, between being an aquatic heron and a terrestrial one. It has two distinct major feeding habitats (shallow water and pasture-prairie), two primary foods (fish and insects), and two seasonal ranges (wet season nesting and dry season non-nesting). In general, during breeding it feeds in wetlands on fish during the wet season, and during non-breeding it feeds more broadly including in wet and dry grasslands on insects and lizards. It appears morphometrically attuned to its dichotomous life, with its bill being relatively shorter and thicker than that of the Great Egret yet relatively larger and thinner than a Cattle Egret's. Similarly its leg and neck proportions appear to be intermediate. It shows behavioural flexibility. It uses the same primary Stand and Walk slowly feeding techniques in both habitats. But it also is flexible in being able to use the Cattle Egret’s Head Swaying and Gleaning and the typical middle-size egret’s ability to use a range of active feeding behaviours such as feeding on the wing in deep water. Its most marked accommodation may be its migratory tendency, which allows populations to shift to accommodate seasonally and annually changing water conditions and its ability to alter its nestes season, geographically and annually, to nest way aquatic prey are available. It is a highly colonial species, nesting in small to large mixed-species aggregations. It is migratory and dispersive as well, moving in response to seasonal changes in wear and rainfall. The Intermediate Heron is adaptable in taking advantage of two alternative ecological situations. Its flexibility is clearly successful, given its situahigh nesting success rate and large population sizes.