Yellow-crowned Night-Heron
Nyctinassa violaceus (Linnaeus)
Ardea violacea Linnaeus, 1758, Syst. Nat. ed. 10(1), p.143: North America = Carolina ex Catesby.
Subspecies: Nyctinassa violacea cayennensis (Gmelin), 1789: Cayenne; Nyctinassa violacea pauper (Sclater & Salvin), 1870: Indefatigable I., Galapagos archipelago; Nyctinassa violacea bancrofti (Huey), 1927: Scamion Lagoon, Lower California; Nyctinassa violacea caliginis (Wetmore), 1946: Panama.
Other names: Crabcracker, Crab-catcher in English; Martinete Coronado, Pederete Enmascarado, Chicuaco Enmascarado Rey Congo, Yaboa Común, Guanabá Real, Crabier Bois, Crabbier, Gaulin in Spanish; Martirão, Savacu-de-coroa in Portuguese; Bihoreau violacé, Crabbier gris, Coq de nuit in French; Geelkruinnachtreiger in Dutch; Krabbenreiher in German.
Description
The Yellow-crowned Night-Heron is a medium blue grey heron with a light cap and cheek and a thick bill.
Adult: The adult has a white crown with a yellow or rusty tinge. The side and back of the head is black. The bill is black with a yellow patch of skin at its base. The lores are yellow grey and the irises are orange. A white streak runs along the side of the head from the bill, below and beyond the eye. The neck is grey. The back and upper wings are grey, patterned by the feathers each having dark center and light margins which form distinctive back stripes. The legs are dull orange yellow.
In breeding, the cap is white and has several long (to 150 mm) back plumes and lack with light margins. The bill becomes glossy black and the lores dark green. The irises turn scarlet and the legs turn scarlet or bright orange.
Variation: Males average larger than females (Watts 1995a). There is geographic variation in the base color of the birds and both geographic and individual variation in bill shape, which is proposed to vary in part in response to the size of local crabs (Watts 1995a). This variation has led to the recognition of up to six subspecies, but some of this variation overlaps variation within the eastern United States population of violacea (Payne 1979).
Five subspecies are recognized. Violacea is the dark form, described above, with broad dorsal streaks and variable bill. Bancrofti has paler plumage (both adult and juvenile) with narrower dorsal stripes, and larger bill. Calignis has a large and thick bill and darker grey plumage, especially juveniles who have a streaked head. Cayennensis is darker, with narrow dark back streaks and slender bill. Pauper is smaller and darker.
Juvenile: The immature is brown streaked with white and cream. The crown is streaked. The bill is black to brown. The iris is yellow orange. The back and wings are spotted in buff white. The under parts are streaked brown and white cream. The legs are dull yellow green.
Chick: The downy chick is white grey, with longer head tufts forming a crown. The eyes are dark yellow. The skin is pink. Upper bill is pink to grey. Lower bill is pale pink to yellow pink with ivory tip. The legs are pink flesh,
Voice: The Yellow-crowned Night-Heron is highly vocal (Bagley and Grau 1979). The “Scaup” call is the often heard, characteristic call of this species. It is similar to the Squawk Call of the Black Crowned Night-Heron but at a higher pitch. It is given throughout the day and night and in all seasons. It is used variously as a contact call, disturbance call, flight call, taking flight call and territorial call. The “Squawk” call is the aggression call, used in the Forward and by the young defending themselves at the nest, as early as 3 weeks of age. The “Ahhh” Call, rendered “ahhh, ahhh”, is a guttural aggressive call. “Whoop” Call is a high-pitched call used in the Stretch. The “Yup” Call, rendered “yup, yup” is a communication between paired birds and used in the Greeting Ceremony. “Huh” call is given during the courtship period when several birds are roosting together and also just prior to the Stretch. Birds use Bill Clappering within the pair bond. Young beg with “chu, chu, chu, chu” or “chee chee, chee, chee”.
Weights and measurements: Length: 55-70 cm; Weight: males average 716 g, females average 649 g.
Field characters
The Yellow-crowned Night-Heron is identified by its grey body and white head and cheeks. The head and neck appear disproportional, such that the head looks relatively large compared to the slim neck supporting it. It flies with slow wing beats, its legs and feet extending beyond the tail.
It is distinguished from the Black Crowned Night-Heron by its white (not black) head and all grey (not black and white) body, and in flight by extended legs. The immature bird is distinguished from the immature Black Crowned Night-Heron by its shorter, deeper bill, longer legs, heavier appearance, erect posture with longer neck, darker brown base color, and fewer and smaller white spots on the back and wings. In flight its legs and feet (not just feet) extend beyond the tail. It is distinguished from the immature Green Backed Heron by its larger size, streaked (not dark) crown, thicker bill, and extensive spotting on back and wings.
Systematics
Once thought to be related to the Nycticorax night herons, this species is distinctive. It is a member of the typical heron group and is likely no more closely related to Nycticorax than it is to the other typical herons, but additional molecular study is desirable.
Range and status
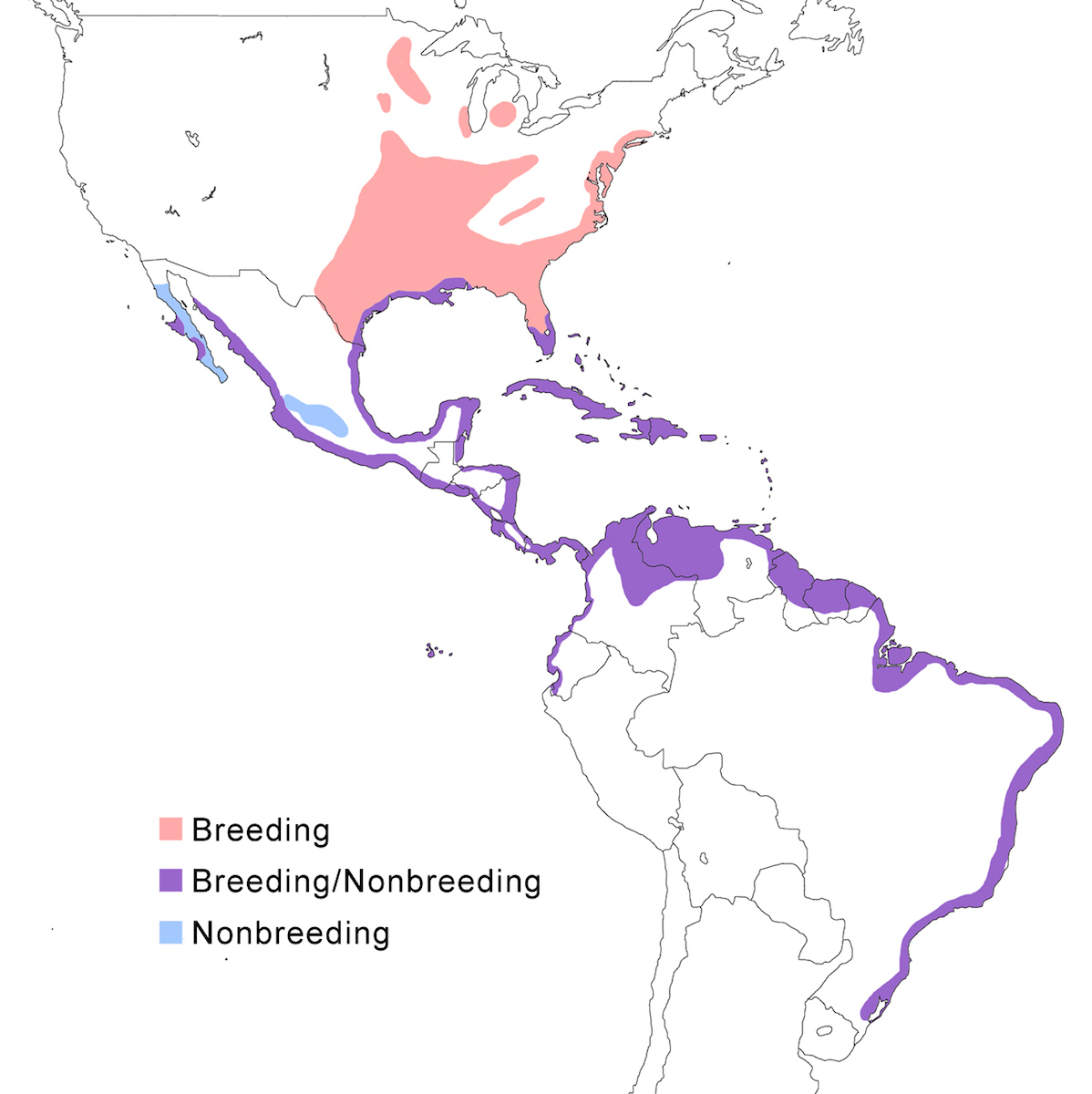
The Yellow-crowned Night-Heron occurs in North America, the West Indies and along coastlines of Central America and South America.
Breeding range: In the United States, violacea breeds from Massachusetts and Connecticut, south along the Atlantic coastal plain to Florida, inland to north Alabama, west Tennessee, west Kentucky, south west Ohio, south Illinois, south Indiana, Missouri, Michigan (McGrath 1988), south west Virginia to south east Tennessee (Williams 1978, Henderson 1985, Robinson 1990), Illinois, Wisconsin, and Minnesota, west to central Oklahoma. It has bred in Michigan and Ontario. It ranges south along the east coast of Mexico, Belize, Honduras, the West Indies from the Bahamas, through the Greater and Lesser Antilles, and Tobago (Payne 1979), and on Bermuda, where it was reintroduced from the Florida population.
Bancrofti occurs in Baja California (Bercerril and Carmone 1997, Whitmore et al. 1999), Socorro Island, Tres Marías Islands, and the Pacific coast of Central America from Mexico (Mazatlán) to Guatemala, El Salvador and Nicaragua (Payne 1979). The night heron occurs along both coasts of Costa Rica (Alvarado pers. comm.) but it is not clear what its subspecies is. Caliginis occurs on the Pacific coast of Panama, Pearl Islands, Cocos Islands, and Caribbean coast of Panama (Almirante, Bocas del Toro, Puerto Olbadia, San Blas), west Colombia, Ecuador, to Peru. The distribution of subspecies in Costa Rica is unclear. Cayennensis occurs from east Panama, east Colombia, coastal and inland Venezuela (Scott and Carbonell 1986), through the Guyanas, Trinidad, north Brazil (extreme north west Amazonas), to central Brazil (Rio Grande do Sul). Pauper occurs on the Galapagos Islands.
Nonbreeding range: The northern population winters in southern parts of the breeding range including south Florida, Gulf of Mexico coasts of Alabama, Mississippi, Louisiana, and Texas, Gulf coastal Mexico, Baja California, Pacific coast of Mexico, the central volcanic belt in Mexico, Nicaragua, Costa Rica, the Caribbean coast of Panama, Colombia, Venezuela, Ecuador and Peru.
Migration: In north North American herons undergo an extensive post breeding dispersal, generally to the north and west. This turns into a southward migration in September. The migratory portion of the population has expanded as the range expanded. Southern limits of their wintering are poorly known, but extend at least to Costa Rica, Cayman Islands, Barbados and Grenadines. Return migration is March. More tropical and island populations appear to be sedentary, but there is little information.
Dispersal records include Canada (south New Brunswick, Newfoundland, Nova Scotia, Clipperton Island, south Quebec, south Ontario, south Saskatchewan, south Manitoba), and north and west United States (Maine, New Hampshire (Petersen 1992), north Wisconsin (Wolinski and Pike 1987), Vermont (Kibbe and Boise 1984), central California, south Arizona (Rosenberg and Stejskal 1992), south New Mexico, Colorado (Kingery 1983b), Montana, North Dakota, Wyoming (Kingery 1983a, Findholt 1984b, Findholt and Berner 1988), Nevada (Kingery 1993).
Status: In the United States, the breeding range expanded northward in the 1940-50’s along both the coast and inland, in part reoccupying its historic range from the 1800’s (Watts 1995a). Range expansion is also occurring in South America (Morales 2000). It is a common bird in its restricted coastal habitat. It is common in the Bahamas, Greater Antilles, Virgin and Cayman Islands and north Lesser Antilles (Raffaele et al. 1998). It occurs in low number in its range in South America but appears to be stable (Morales 2000). It is common in the Galapagos.
Habitat
This is a fundamentally a coastal heron, occurring inland only where crustaceans are readily available. It prefers habitats with significant edges along which to feed. Its principal habitats are coastal marshes, swamps, lagoons and shores and inland river basin swamps and marshes. Its specific feeding habitats are the edges of tidal mangrove swamps, tidal mudflats, rocky shores including tide pools, sandy shores including feeding in the surf, banks of slow moving rivers, swamps, ponds, reservoirs. It feeds in upland sites along arid island shores, pasture, lawns, parks, and ploughed fields. Nesting habitat is usually forests and wooded areas near suitable foraging sites, usually within 1.5 km.
Foraging
It feeds by Standing and waiting for prey in either Upright or in Crouched posture. This takes a preponderance of its feeding time, 77% in Florida and 64% in Missouri (Rodgers 1983, Laubhan et al. 1991). In Standing, the bird waits patiently for a prey item to appear, and if it does not it moves by walking or hopping to a new site. In feeding for crayfish, birds would target a burrow and wait for the crayfish to emerge (King and Leblanc 1995).
It also feeds by Walking, usually along the shore or in shallow water in Crouched posture with its head either extended or retracted. It feeds carefully and deliberately. The balance of time spent in Walking and Standing differs with habitat; Walking is used more on vegetated than on unvegetated shores (Rodgers 1983). It uses Head Swaying and Neck Swaying to observe prey. It catches prey by a Bill Lunge. It Pecks at small prey, merely Grasping them. It will also Run, presumably between feeding sites (Calyton 1985).
It is primarily a solitary forager, and defends its feeding space. It feeds in loose aggregations at sites where prey is abundant. It also defends its feeding space in these aggregations, leading to the birds being well spaced. It is characteristic for Yellow Crown Night-Herons when feeding together to be uniformly spaced at distances on the order of 5-10 m.
Given the difficulty of capture and hard shells of its characteristic prey, learning appears to be an important characteristic of its feeding. Adults spend less time handling prey than juveniles, indicating that some skill development is required (Laubhan et al. 1991). In one study juveniles took more easily captured tadpoles whereas adult caught larger, burrowing crayfish (Laubhan et al. 1991), again suggesting the importance of learning. Young Walk more than do adults in feeding (Rodgers 1983).
Handling the hard-shelled, pincer-equipped prey is a fundamental component of the feeding of this species. Small prey are swallowed whole. But a large prey is repeatedly grabbed and shaken. The heron will jab its bill through the carapace, which appears to stun the crab (Watts 1995a). In shaking, the claws and legs are broken off. The body is further broken up or eaten whole. The legs are then eaten. Size and defensive behavior and morphology of prey influence handling time (Reigner 1982a). Larger prey are often taken away from the feeding site for handling. The undigested shells are cast as pellets.
Although called a night heron, it is not dependently nocturnal. It does feed at night, but also crepuscularly and during the day. Daytime feeding occurs especially on islands and especially to take advantage of tidal cycles, feeding for several hours around low tides (Austin 1995, Watts 1995a). It is a visual forager by both day and night (Clayton 1985). Nighttime feeding is often done around artificial lights.
This heron is a crab specialist. It feeds principally on crabs and other crustaceans such as crayfish, in fresh water. Is crustacean diet is varied including mud crabs (Uca, Rithropanopeus, Panopeus, Ucides), shore crabs (Pachygrapsis, Grapsis), land crabs (Gecarcinus), mangrove and marsh crabs (Goniopsis, Sesarma), swimming and shallow water crabs (Callinectes, Ovalipes, Carcinus, Cancer, Hyas), beach crabs (Ocypode), mole crabs (Emerita), crayfish (Procambarus, Cambarus).
It can eat relatively large crabs but consumes smaller crabs more efficiently than large ones. Size and species taken vary geographically. Its dependence on crustaceans can be seen in various feeding studies. As a percent of the diet, crustaceans were 79% in south United States, 74% in Louisiana, 97% and 91% in north east United States, 76% in Missouri, 97% in Bermuda (Watts 1995a).
It also takes other food (Riegner 1982b, Niethammer and Kaiser 1982, Watts 1988). These prey include leeches, earthworms, marine worms, centipedes, snails, amphipod (Ampithoe), insects (grasshoppers, crickets, katydid, water beetles, water bugs, soldier fly), scorpions, tadpoles (Rana), frogs, lizards, snakes, fish (Anguilla, Micropterus, Lepomis, Notropis, Fundulus), small mammals (Mus, Peromyscus, Sylvilagus).
The use of alternative prey appears to be related to their occurrence within the same habitat as the principal crustacean prey for the population (Watts 1988). The use of alternative prey is important for juveniles, who are less efficient than adults at catching crabs. Also seasonal reduction in crab abundance leads the birds to switch to insects (Clayton 1985).
Breeding
The breeding season in the United States, Trinidad and Surinam is March–July. In the northern parts of the range, breeding season is constrained by date of crab emergence, which depends on temperature (Watts 1995a). In tropical areas, nesting is associated with the rainy season but has been reported over much of the year.
It nests in various situations, always near water given the specificity of its prey dependence. On the coast, nesting is generally on islands including barrier islands, artificial dredge material islands, and in coastal mangroves. Inland, they nest in swamps and uplands near lakes, rivers, and creeks. It also nests in human dominated environments, such a residential areas. They use a variety of trees, often nesting near to or over the water Trees used include Rhizophora, Pinus, Quercus, Liquidambar, and bushes include Baccharis, Morus, Myrica, Celtis. On dry islands they can nest on the ground, rock ledges, and cactus.
The most typical situation is for the species to nest in scattered pairs or in small loose colonies. The average colony size in Virginia and in Missouri was about 4 nests (Laubhan and Reid 1988, Watts 1995a). In Virginia, a quarter of all pairs nested singly In larger colonies they tend to occur at low density, mixed among the other species. Total nests may number in the low hundreds. Seldom do more than one pair nest in a tree. However, larger colony sites may be persistently used for decades.
The nest is a platform with a slight depression, made of twigs and variably lined with grass, leaves, or moss. Dimensions appear to be variable, but surprisingly little documented. Reported nests range from 1.25 to 0.5 m wide. Size may depend on the availability of sticks and the reuse of old nests. In northern latitudes they are begun about 10 days after arrival at the nesting site. Several nests may be started before the final one is chosen. Nests are constructed by both birds, but it is more the case that the male gathers sticks and the female works to put them together. Nests are repaired throughout nesting by both birds. Rebuilding after nest failure may be part of the renesting cycle (Watts 1995a).
Nest height varies, from the ground to 35 m (Laubhan and Reid 1991) depending on the situation. High nests on branches out from the trunk may be the most typical situation (Laubhan and Reid 1991). On islands, they my be on shorter plants, on the ground, or even in caves.
Males claim and defend advertising sites and display in them (Bagley and Grau 1979). Circle Flights and other kinds of flying around the colony are common at this time. In the Circle Flight, the wing beats often are flapped to produce a loud “womp, womp” sound. They aggressively defend their sites using Forward, Pursuit Flights, and Supplanting Flights. The main display is the Stretch, given by the male and sometimes by the female in response. Just prior to the Stretch the birds may give a Huh Call. In the stretch, the bird extends its head up in a slow, steady movement; bill is horizontal; head is lowered to touching the back; back plumes are erected to form a fan behind the head; legs are flexed; the bill is then tilted up and a Whoop Call is given. Bill Clappering is common between the paired birds at the nest site, as is Preening and Standing. Circle Flights also are common at this time. Copulation occurs on the nest sites. After pairing, the Greeting Ceremony is used upon the approach of one of the pair. It consists of Crest Raising and the greeting birds giving the Yup Call.
Eggs are pale green blue. Size averages are 51 x 37.4 mm in the United States, 47.5 x 35 mm in Trinidad, 50 x 36 in Tobago, and 49.1 x 34.3 mm in Surinam. Clutch size is 4-5 in northern populations (averages are 2.9, 3.5, 4.3), 2-4 in tropics, overall range 2-8. Birds renest after egg loss but in the north they are not inherently double brooded as was long thought (Watts 1995a). Both birds incubate beginning with the first egg. Birds attend the nest continually after laying. The nonincubating parent spends much time staying near the nest early in the incubation period, presumably for guarding. They sit on eggs up to 88%of the time (Bagley and Grau 1979). Incubation period is 21-25 days. More information is needed; one definitive example was 24-25 days (Weeks 1976).
Hatchlings are semialtricial and nidicolous. Eyes open on day 1. Both parents attend and care for the chicks. They feed the young by regurgitation onto the center of the nests. Grasping of the parent’s bill does not seem to be important in this species (Bagley and Grau 1979). Parents recognize their young. After fledging, the young return to the nest to be fed. Growth is rapid over five weeks (Watts 1995a). Pinfeathers emerge on day 5. Flight feathers appear on day 12 (McVaugh 1975). Over the development period, the irises become orange yellow, legs yellow green, skin grey green. Young fledge in about 30 days and can fly independently at 50 days. At independence the young are below adult size.
Nesting success varies, 3.6 in Alabama, 2.25 in Florida, 2 in Bermuda, 1.7 young/nest in Virginia. Nesting success in Virginia varied from 0.6 to 3.1 among years. Success depends on the extent of losses, but also timing in that later losses are not replaced by renesting. Egg and nest predators include crows (Corvus), raccoon (Procyon), opossum (Didelphis), and domestic dogs (Canis) and cats (Felis).
Population dynamics
Typically Yellow-crowned Night-Herons first nest at two years when they acquire full adult plumage. However birds in juvenal plumage do nest successfully. Immature birds accounted for less than 0.5% of breeding population (Watts 1995a, b). No information exists on life span or survivorship. Adults may be killed by owls (Bubo) and hawks (Buteo), the latter has been observed drowning a heron (Richter 1985). Because they use human dominated areas, young are susceptible to being killed by dogs and cats.
Conservation
The Yellow-crowned Night-Heron is a widespread, even if not a locally very abundant species. It has expanded its range in the last century. It is considered a species of concern in some states in the USA, due its peripheral status and perhaps a lack of information. But overall the population appears stable in both North and South America, to the extent that is known. Quantitative trend data are for the most part lacking. Certainly the most important conservation issue is habitat protection. The dependence of the species on a small array of prey types and very selective habitat choice makes it a candidate for special consideration within habitat management plans. For example, the timing and speed of water level draw down of wetlands may affect nesting viability (Laubhan and Reid 1991). Loss and alteration of mangrove forests and other coastal habitats, especially in the southern portion of its range, is a long-term threat. In the USA nesting near and even over houses, roads, and driveways leads to conflict (Watts 1995a). Disturbance in colony sites is an important issue, and needs to be managed in sites near people.
Research needs
Studies are needed on the demography, movements, survival, and distribution of juvenile Yellow-crowned Night-Herons (Watts 1995a). Feeding habits, maturation of feeding efficiency, potential residency outside the breeding range, survival, and site fidelity are all issues that deserve detailed scrutiny. Additional study is needed on breeding phenology, particularly on the relationships among weather, prey emergence, and breeding cycle in more northern latitudes and other factors influencing prey availability elsewhere.
Overview
The Yellow-crowned Night-Heron is an intriguing species, in that it shows the most specialized development among crab-eating herons. Its stout, strong bill is used for catching and handling these hard-bodied and somewhat threatening prey. That the bill is geographically variable is thought to be an evolutionary response to the size of prey. Island populations eat relatively large prey, such as land crabs. Other populations eat smaller and softer prey, such as inland crayfish. Its dark coloration is cryptic, but it is not solely a night heron. In fact, it feeds whenever is best for the prey available. Although not thoroughly studied, its nesting season appears to be geared toward the timing of availability of crabs, whether based on rainy season or spring temperatures. Developing skills to capture and handle crustaceans appears to take some time. Very few birds attempt to nest before year 2. Younger birds are less efficient, more affected by having other birds nearby, and take easier to catch (and probably not as profitable) prey. Much is yet unknown about what may be the distinctive ecology of juvenile birds. This is a successful species despite its narrow niche. It is widespread and not declining seriously anywhere in its range. It is likely that its flexibility and adaptability are the keys to its success.