Flush-pursuit foraging behavior by a Great Egret (Ardea alba)
Abstract
Among wading birds, 39 foraging behaviors have been described including flushing followed by the immediate pursuit/capture of prey. However, this behavior has not been observed in the Great Egret. Here we report on a new foraging behavior for this species. On 9 October 2022, we recorded at Clinton Lake, Kansas, U.S.A., an adult Great Egret (Ardea alba) grabbing and lifting a partially-submerged floating leafed Buttonbush branch (Cephalanthus occidentalis) out of the water, shaking and dropping the branch, and then rapidly stabbing for prey away from the dropped branch. The egret repeated this sequence of behaviors twice over <1 min. To further assist in the interpretation of this observation, we conducted a pool study of the response of a common prey species to the lifting, shaking and dropping of a Buttonbush branch. In 9 of 9 trials, Green Sunfish (Lepomis cyanellus), an important prey item of the Great Egret, rapidly swam away from the branch when lifted, shook and dropped. These observations and photographs combined with a pool study provide supporting evidence for a previously undescribed form of foraging behavior for the Great Egret that we term Flush-pursuit.
Key words: branch, Flush-pursuit, foraging behavior, Great Egret, pool study.
Introduction
In an extensive review of the feeding ecology of wading birds, Kushlan (1978) described 38 foraging behaviors. Kelly et al. (2003) updated this account for nine species of wading birds and included an additional foraging behavior.
Here we report an undescribed foraging behavior for the Great Egret (Ardea alba) that we term Flush-pursuit and provide supporting evidence for this observation through a pool study that examined the response of a common prey species to the lifting, shaking and dropping of a leafed branch.
Methods
On 9 October 2022, GWS observed and photographed an adult Great Egret during a 6 min feeding bout at Clinton Lake, Douglas County, Kansas, U.S.A., in the Bloomington Public Use Area west of the boat ramp 6 (38° 54′ 49.902″ N, 95° 22′ 16.728″ W). The bird was observed and photographed from a distance of approximately 50 m using an 800 mm lens.
To further assist in the interpretation of the observed Great Egret’s foraging behavior, we conducted a pool study, between 10-12 October 2023, of the response of a common prey species to the lifting, shaking and dropping of a leafed Buttonbush (Cephalanthus occidentalis) branch. We placed 10 Green Sunfish (Lepomis cyanellus), 8-12 cm in length, in a 4.5 m x 2.5 m plastic-lined pool whose edge was lined with small rocks for approximately two-thirds of the pool’s perimeter. We selected the Green Sunfish as a model species for several reasons. Firstly, it is the most abundant sunfish in aquatic bodies in eastern Kansas (Cross and Collins 1995); secondly, it is also abundant in shallow water in lakes in eastern Kansas (GWS, pers. obs.); thirdly, sunfish (Lepomis spp.) are important prey items of the Great Egret in other areas in North America (Frederick et al. 2009); and fourthly, live Green Sunfish are one of two fish species, the other being the Bluegill (Lepomis macrochirus), that can be legally moved in Kansas between water bodies that do not contain “aquatic nuisance species”. We collected live Green Sunfish from a farm pond 120 m away from the artificial pool. The water depth in the artificial pool was maintained between 8-12 cm so as to mimic the water depth in Clinton Lake where the Great Egret was observed.
In the deeper portion of the pool, we placed two leafed Buttonbush branches, 50 cm in length. One end of the branches was braced at the bottom of the pool by several small rocks. The opposite end of the branches was held partially submerged and parallel to the surface of the water by a monofilament line that bound the upper portion of the two branches. The monofilament line was in turn attached to a pole 2 m in length that was suspended 1.5 m above the water.
After introduction of the sunfish to the pool, we allowed the sunfish to acclimate for >18 hr before initiating the manipulative trials. We conducted 9 video-recorded trials. During a trial, the Buttonbush branches were lifted, slightly shook and dropped over a 2 sec period. We recorded (1) whether the Green Sunfish moved away from the Buttonbush branches or remained stationary; (2) the number of sunfish that dispersed away or remained stationary under the Buttonbush branches; (3) the elapsed time between when the Buttonbush branches were lifted and when the sunfish moved away from under the branches; and (4) the distance that the sunfish moved after the branches were manipulated. We used the length of the Buttonbush branches (50 cm) as a metric to estimate in the video clips the distance a sunfish moved from the base of the submerged branches to where the sunfish temporarily stopped after the branches were lifted and shook.
Observation
The Great Egret was initially observed at 08:32 hr walking slowly in the water ~1 m from the water’s edge and parallel to the shoreline in a typical Walk-slowly (Kelly et al. 2003) foraging mode. The egret walked for about 25 m in a northwesterly direction along the water’s edge and then turned and walked in the opposite direction for about 12 m, again parallel to the shoreline. Between 08:32 hr and 08:36 hr, the egret was observed to stab and capture several small fish. However, because of the speed with which the egret stabbed and captured the small fish, it was not possible to identify either the species of fish or the number of fish that were captured during this initial 4 min observation.
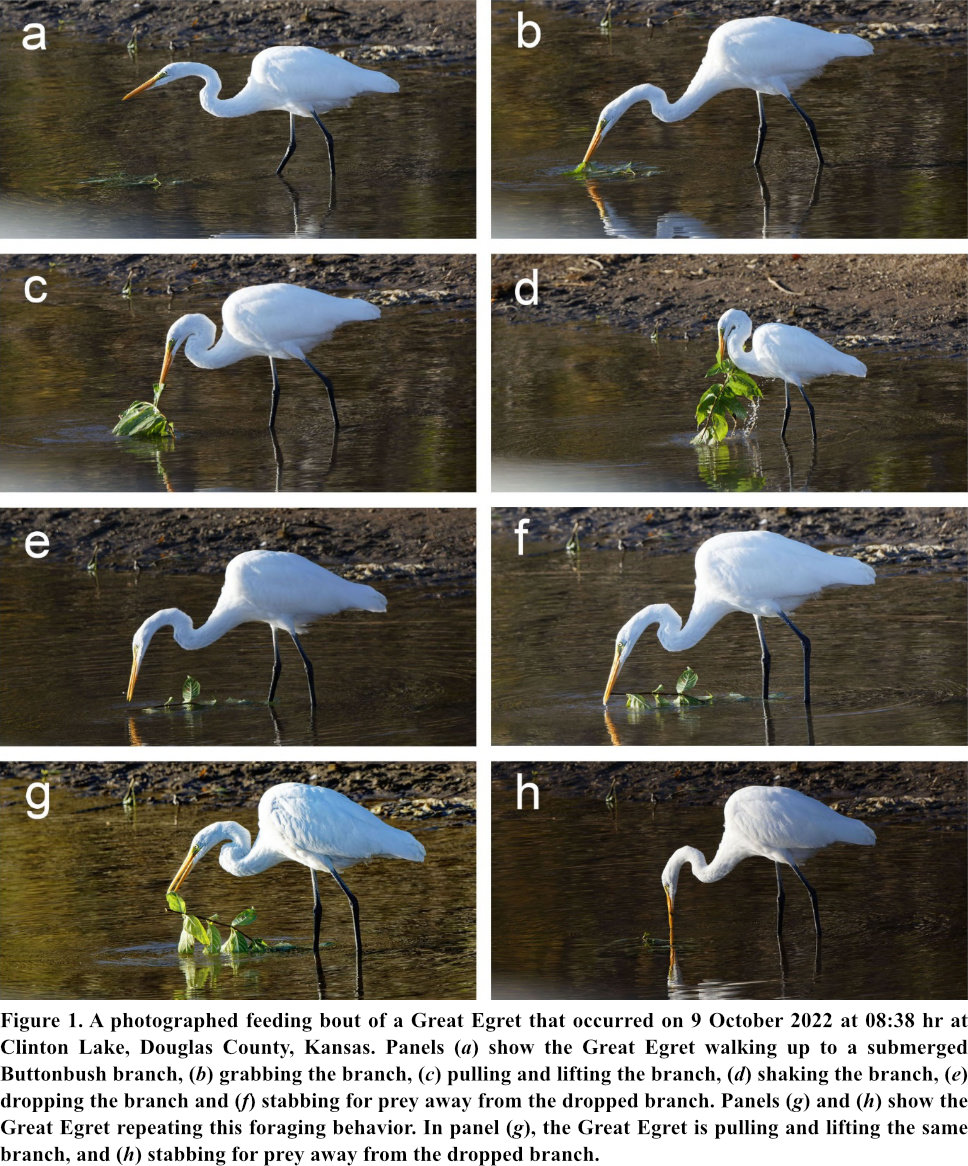
At 08:37 hr, the Great Egret was observed and photographed catching a Gizzard Shad (Dorosoma cepedianum), ~9 cm in length close to the water’s edge. After capturing the gizzard shad, the egret at 08:38 hr turned and walked ~1.5 m diagonally away from the shore and stopped in water 8-10 cm in depth adjacent to a floating partially-submerged Buttonbush branch, ~47 cm in length (Fig. 1a). The egret bent over, grabbed the leafed branch, pulled and lifted it, shook it laterally and dropped the branch back into the water (Fig. 1b-e). After dropping the branch, the egret rapidly stabbed for prey ~10-15 cm away from the dropped branch (Fig. 1f). The egret then repeated again this sequence of behaviors: grabbing, pulling, lifting, shaking and dropping the same branch, and then rapidly stabbing for prey ~20-30 cm away from the dropped branch (Fig. 1g, h). These two observed foraging events, which involved lifting, shaking and dropping a branch to presumably expose and/or disperse prey (flush prey), occurred over <1 min. It was not possible, however, to observe whether the egret was successful in capturing any prey during the two foraging events. After these two foraging events, the egret turned and walked back to shore and proceeded to walk slowly along the shoreline in an easterly direction.
Pool study
Ecologists are increasingly employing pool and pond studies to study fish movement and establish cause-and-effect relationships (Brönmark et al. 2023). In addition to allowing us to interpret the observed foraging behavior of the Great Egret, an important objective of the pool study was to assess whether fish disperse rapidly away from branches when lifted, shook and dropped.
During the acclimation period, as well as between trials, we observed the sunfish sheltering in rock crevasses along the edge of the pool as well as below the partially submerged Buttonbush branches.
The mean number of sunfish sheltering under the Buttonbush branches immediately before the trials was 3.5 ± 1.7 SD per trial. In 9 of 9 trials, all of the Green Sunfish that were sheltering under the Buttonbush branches rapidly swam away (i.e., flushed) from under the branches when lifted, shook and dropped rather than remaining stationary (Video 1). This pattern was highly significant (sign test, P < 0.001). The mean elapsed time between when the first sunfish moved after the branches were lifted and shook was 0.7 ± 0.5 SD sec, and the mean distance between the base of the submerged Buttonbush branches and where the sunfish temporally stopped after the branches were manipulated was ~38 ± 8 SD cm.

Discussion
Here we present evidence, observational, photographic and pool, for a previously undescribed form of foraging behavior by a Great Egret which we term Flush-pursuit. The pool study provides supporting evidence for the description and interpretation of this foraging behavior. In the pool study, a common prey species, the Green Sunfish, rapidly dispersed away from under the branches (i.e., flushed) after the branches were lifted, shook and dropped, and then temporally stopped a short distance from the dropped branches.
Flush-pursuit foraging behavior has been previously described among other vertebrate taxa. Among terrestrial insectivorous birds, Flush-pursuit foraging behavior is known among many species including the American Redstart (Setophaga ruticilla), Northern Mockingbird (Mimus polyglottos), Ruddy-tailed Flycatcher (Terenotriccus erythrurus), Mountain Plover (Charadrius montanus), Hooded Warbler (Setophaga citrina), Dendrocincla spp., Rhipidura spp., Monarcha spp., Myiobius spp. and Myioborus spp. (Remsen and Robinson 1990, Jabłoński 2002, Mumme 2002, 2014, Alberta Sustainable Resource Development 2003, Dharmarathne and Mahaulpatha 2015, Mansor et al. 2020).
Among marine, aquatic and terrestrial mammals, Flush-pursuit has been described in Wendell’s Seal (Leptonychotes weddellii), American Water Shrew (Sorex palustris) and Dingo (Canis familiaris) (Davis et al. 1999, Catania et al. 2008, Fleming et al. 2022).
Among wading birds, the key elements of Flush-pursuit foraging behavior – flushing followed by the immediate pursuit/capture of prey – has been described, although not termed as such, in descriptions of Foot-stirring in the Snowy Egret (Egretta thula), Reddish Egret (Egretta rufescens) and Green Heron (Butorides virescens) (Meyerriecks 1959, 1966), as well as in descriptions of Foot-raking in the Snowy Egret and Little Blue Heron (Egretta caerulea) (Meyerriecks 1959, 1971). Thus, among wading birds Flush-pursuit foraging behavior is not unique to the Great Egret.
Our observations and photographs combined with a pool study of a common prey species provide supporting evidence for a previously undescribed form of foraging behavior – Flush-pursuit – in the Great Egret.
Literature Cited
Alberta Sustainable Resource Development. 2003. Status of the Mountain Plover (Charadrius montanus) in Alberta. Alberta Wildlife Status Report No. 50. Alberta Sustainable Resource Development, Fish and Wildlife Division, and Alberta Conservation Association, Edmonton, Alberta, Canada. [online].
Brönmark, C., G. Hellström, H. Baktoft, L. A. Hansson, E. S. McCallum, P. A. Nilsson, C. Skov, T. Brodin and K. Hulthén. 2023. Ponds as experimental arenas for studying animal movement: current research and future prospects. Movement Ecology 11: 1-15.
Catania, K. C., J. F. Hare and K. L. Campbell. 2008. Water shrews detect movement, shape and smell to find prey underwater. Proceedings of the National Academy of Sciences of the United States of America 105: 571-576.
Cross, F. B. and J. T. Collins. 1995. Fishes in Kansas. 2nd edition. University of Kansas Press, Lawrence, Kansas, U.S.A.
Dharmarathne, W. D. S. C. and W. A. D. Mahaulpatha. 2015. Foraging behavior of endemic Dull-blue Flycatcher (Eumyias sordidus) in tropical montane cloud forest habitats of Sri Lanka. International Journal of Science and Research 7: 111-118. [online].
Davis, R. W., L. A. Fuiman, T. M. Williams, S. O. Collier, W. P. Hagey, S. B. Kanatous, S. Kohin and M. Horning. 1999. Hunting behavior of a marine mammal beneath the Antarctic fast ice. Science 283: 993-996.
Fleming, P. A., A. M. Stobo-Wilson, H. M. Crawford, S. J. Dawson, C. R. Dickman, T. S. Doherty, P. J. S. Fleming, T. M. Newsome, R. Palmer, J. A. Thompson and J. C. Z. Woinarski. 2022. Distinctive diets of eutherian predators in Australia. Royal Society Open Science 9: 220792. [online].
Frederick, P. C., M. G. Spalding, M. S. Sepúlveda, G. E. Williams, L. Nico and R. Robins. 2009. Exposure of Great Egret (Ardea albus) nestlings to mercury through diet in the Everglades ecosystem. Environmental Toxicology and Chemistry 18: 1940-1947.
Jabłoński, P. G. 2002. Searching for conspicuous versus cryptic prey: search rates of flush-pursuing versus substrate-gleaning birds. Condor 104: 657-661.
Kelly, J. F., D. E. Gawlik and D. K. Kieckbusch. 2003. An updated account of wading bird foraging behavior. Wilson Bulletin 115: 105-107.
Kushlan, J. 1978. Feeding ecology of wading birds. Pages 249-296 in Wading birds (A. J. Sprunt, J. Ogden and S. Winckler, eds.). National Audubon Society, New York, New York, U.S.A.
Mansor, M. S., S. M. Nor and R. Ramli. 2020. Shifts in foraging behaviour of heterospecific flocking birds in a lowland Malaysian rainforest. Behavioural Processes 180: 104229. [online].
Meyerriecks, A. J. 1959. Foot-stirring feeding behavior in herons. Wilson Bulletin 71: 153-158.
Meyerriecks, A. J. 1966. Additional observations on “foot-stirring” feeding behavior in herons. Auk 83: 471-472.
Meyerriecks, A. J. 1971. Further observations on use of the feet by foraging herons. Wilson Bulletin 83: 435-438.
Mumme, R. L. 2002. Scare tactics in a Neotropical warbler: white tail feathers enhance flush-pursuit foraging performance in the Slate-throated Redstart (Myioborus miniatus). Auk 119: 1024-1035.
Mumme, R. L. 2014. White tail spots and tail-flicking behavior enhance foraging performance in the Hooded Warbler. Auk 131: 141-149.
Remsen, J. and S. K. Robinson 1990. A classification scheme for foraging behavior of birds in terrestrial habitats. Studies in Avian Biology 13: 144-160.



